Question
Question: For gaseous state, if most probable speed is denoted by \({C^*}\), average speed by \[\overline C \]...
For gaseous state, if most probable speed is denoted by C∗, average speed by C and root mean square speed by C, then for large number of molecules the ratio of these speeds are:
a- C∗: C : C = 1.128 : 1.225 : 1
b- C∗: C : C = 1 : 1.128 : 1.225
c- C∗: C : C = 1 :1.125 : 1.128
d- C∗: C : C = 1.125 : 1.128 : 1
Solution
The given three representations of velocity are related to each other by following formula:
C∗: C : C = M2RT : πM8RT: M3RT
Complete answer:
Kinetic Molecular Theory explains the macroscopic properties of gases and can be used to calculate the different kinds of gaseous speeds. Measuring the velocities of particles at a given time results in a large distribution of values; some particles may move very slowly, others very quickly, and because they are constantly moving in different directions, the velocity could equal zero.
-Most probable speed (C∗): The most probable speed is the speed most likely to be possessed by any molecule of the same mass m in the gaseous system and corresponds to the maximum value. And it is given by the following formula:
C*= M2RT
-Average speed (C): Movement of gaseous molecules is in random speeds and in random directions. The Maxwell-Boltzmann Distribution describes the average speeds of collection gaseous particles at a given temperature. And it is given by:
C=πM8RT
-Root mean square speed (C or Crms): As the name represents roots of mean of squares of the velocities is known as the root-mean-square (RMS) velocity, and it is represented as follows:
C or Crms=M3RT
On comparing these three velocities:
C∗: C : C = M2RT : πM8RT: M3RT
=2:π8:3
=1 : 1.128 : 1.225
So, the correct option is (B) 1: 1.128: 1.225 .
Note:
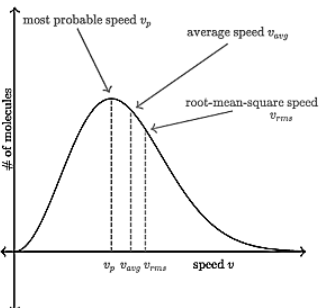
Most probable speed, average speed, and root mean square speed are related to each other by the formula stated above.Their relation can be expressed as a graph as shown below: