Question
Question: For first order kinetics, Co is the initial concentration. If 25% of reactant decomposes in t25% the...
For first order kinetics, Co is the initial concentration. If 25% of reactant decomposes in t25% then, concentration of reactant left after n t25%
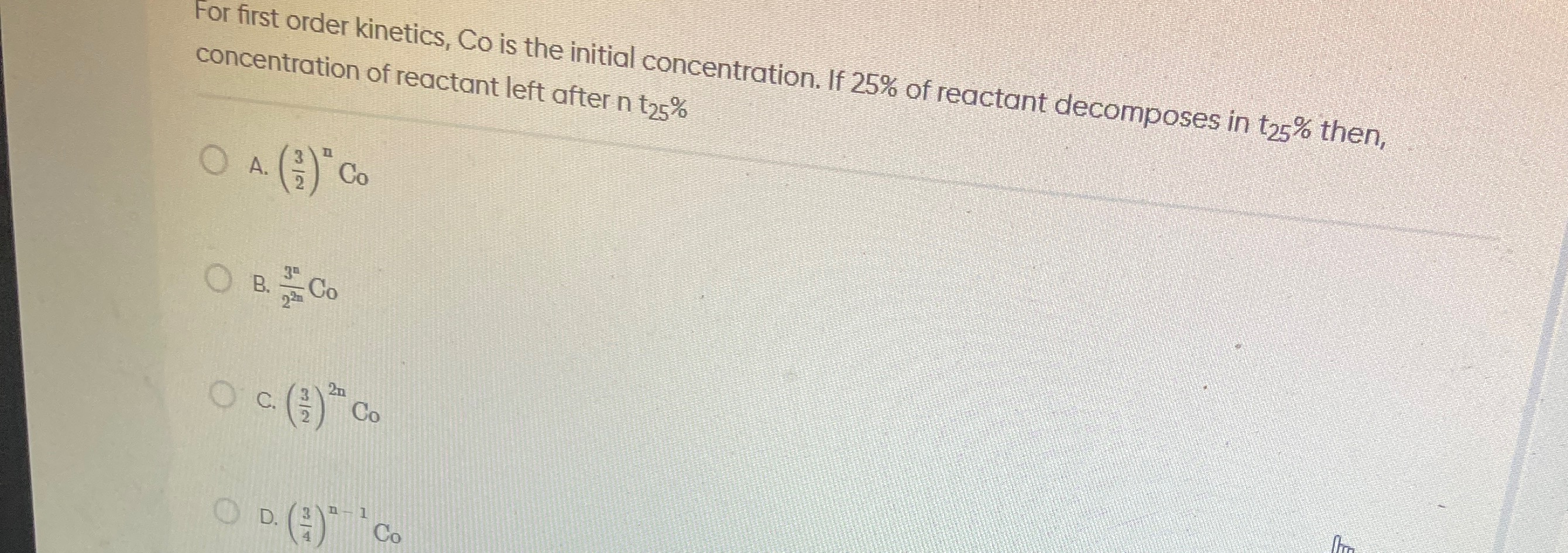
(23)n Co
22n3nCo
(23)2n Co
(43)n−1 Co
22n3nCo
Solution
The problem asks for the concentration of a reactant left after n⋅t25% for a first-order reaction, where t25% is the time taken for 25% of the reactant to decompose.
1. Integrated Rate Law for First-Order Reactions:
For a first-order reaction, the integrated rate law is given by:
kt=ln(CtC0)
where:
- k is the rate constant
- t is the time
- C0 is the initial concentration
- Ct is the concentration at time t
Alternatively, it can be written as:
Ct=C0e−kt
2. Determine the relationship involving t25%:
Given that 25% of the reactant decomposes in t25%, it means that 75% of the reactant remains.
So, at t=t25%, the concentration remaining Ct25% is 0.75C0.
Ct25%=43C0
Substitute this into the integrated rate law:
k⋅t25%=ln(43C0C0)
k⋅t25%=ln(34)
3. Calculate the concentration left after n⋅t25%:
Let the total time be T=n⋅t25%. We want to find the concentration CT at this time.
Using the integrated rate law:
k⋅T=ln(CTC0)
Substitute T=n⋅t25%:
k⋅(n⋅t25%)=ln(CTC0)
n⋅(k⋅t25%)=ln(CTC0)
Now, substitute the value of (k⋅t25%) from step 2:
n⋅ln(34)=ln(CTC0)
Using the logarithm property alnb=lnba:
ln((34)n)=ln(CTC0)
Since the natural logarithms are equal, their arguments must be equal:
(34)n=CTC0
To find CT, rearrange the equation:
CT=C0⋅(34)n1
CT=C0⋅(43)n
The derived expression matches option B.
