Question
Question: For an orbital in $B^{+4}$ radial function is : $R(r) = \frac{1}{\sqrt[3]{Z}}(\frac{Z}{a_0})^{\frac{...
For an orbital in B+4 radial function is : R(r)=3Z1(a0Z)2596(4−σ)σe−σ/2
where σ=na02Zr and a0=0.529A˚;Z=atomic number,r=radial distance from nucleus.
The radial node of orbital is at distance ......... A˚ from nucleus.
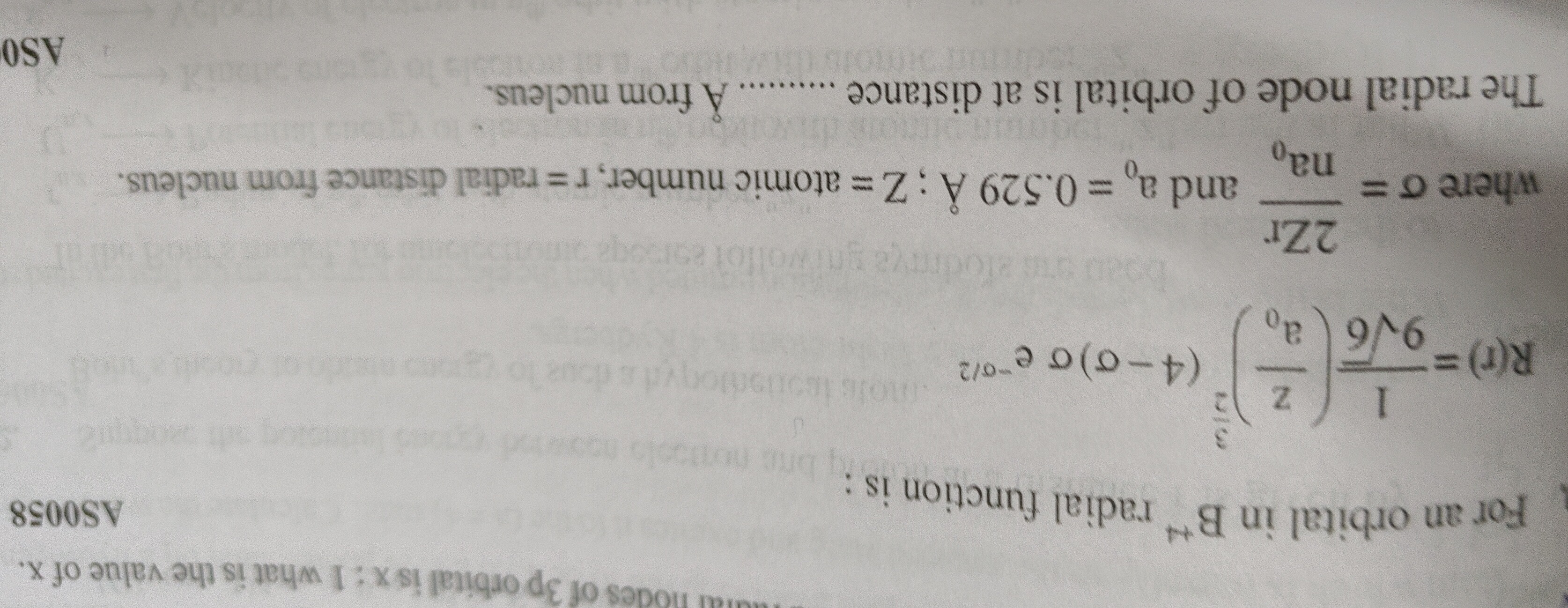
Answer
0.6348
Explanation
Solution
The given radial function is proportional to σ(4−σ)e−σ/2. Comparing with the general form σlLn−l−12l+1(σ)e−σ/2, we deduce l=1 and Ln−23(σ)∝(4−σ), which implies n−2=1, so n=3. The orbital is 3p. The number of radial nodes is n−l−1=1. Nodes occur where the radial function is zero for r>0. The roots of σ(4−σ) are σ=0 and σ=4. Thus, the radial node is at σ=4. For B+4, Z=5 and n=3. Substituting σ=4 into σ=na02Zr gives 4=3a02×5×r, yielding r=1.2a0. With a0=0.529A˚, r=0.6348A˚.
