Question
Question: For an octahedral complex, which of the following d-electron configurations will give maximum CFSE? ...
For an octahedral complex, which of the following d-electron configurations will give maximum CFSE?
(A) High spin d6,−0.4Δo d6,−0.4Δo
(B) Low spin d4,−1.6Δo d4,−1.6Δo
(C) Low spin d5,−2.0Δo
(D) High spin d7,−0.8Δo
Solution
The value of CFSE is more in the given options for the option (C). However it can be understood from the calculation.
Complete step by step answer:
Crystal field splitting energy, CFSE for octahedral complexes is calculated from the following formula,
[(−0.4×5)+(+0.6×2)]Δo
Let us calculate the CFSE for all the given electronic configurations. Let us consider,
- Option (A), in the high spin d6 configuration, there will be 4 t2g electrons and 2 eg electrons. The CFSE is [(−0.4×4)+(+0.6×2)]Δo
=[−1.6+1.2]Δo
=−0.4Δo
- Option (B), in low spin d4 configuration, there will be 4 t2g electrons and 0 eg electrons and its CFSE is = [−0.4×4]Δo
= −1.6Δo
- Option (C), for low spin d5 configuration, the number of t2g electrons are 5 and eg are 0, its CFSE is,[−0.4×5]Δo=−2.0Δo
- Option (D), in the high spin d7 configuration, the number of t2g electrons are 5 and eg are 2, its CFSE is,
=[(−0.4×5)+(+0.6×2)]Δo
=[−2.0+1.2]Δo
=−0.8Δo
From the above obtained values of Crystal field stabilization energies, the value for low spin d5 configuration is maximum which is, −2.0Δo.
The correct answer is option “C” .
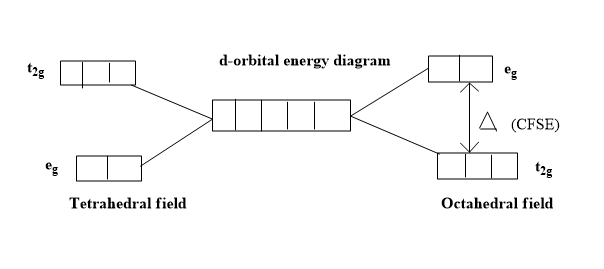
Additional Information : The stabilization energy gained by the complex by filling electrons in lower energy t2g orbitals of octahedral complexes is known as crystal field stabilization energy of octahedral complexes. t2g and eg orbitals are sets of orbitals resulting from splitting of the degenerate d-orbitals. The d-orbitals in which their lobes are oriented along x and y axes are named as eg orbitals; these are also called double degenerate orbitals. The d-orbitals whose lobes are oriented in between x, y, z axes are named as t2g orbitals. These are also called triple degenerate orbitals. These two sets of orbitals have differences in their energies.
Note: The electrons are filled in the orbitals for calculating CFSE by considering whether the given complex is a low spin complex or a high spin complex. In a low spin complex pairing of electrons takes place whereas in a high spin complex, all the degenerate orbitals are filled by one electron each and then pairing occurs.
