Question
Question: For, a zero-order reaction, the plot of concentration of reactant versus time is: (Intercepts refe...
For, a zero-order reaction, the plot of concentration of reactant versus time is:
(Intercepts refers to the concentration axis)
A. Linear with +ve slope and negative intercept
B. Linear with -ve slope and zero intercept
C. Linear with -ve slope and non-zero intercept
D. Linear with +ve slope and non-zero intercept
Solution
Zero-order reaction is a chemical reaction where the rate of reaction does not vary with the increase or decrease in the concentration of the reactants. Therefore, the rate of these reactions is always equal to the rate constant of the specific reactions (since the rate of these reactions is proportional to the zeroth power of reactants concentration).
Complete step by step answer:
The differential form of zero-order reaction can be written as:
⇒Rate = dt −dA=k[A]0=k
Where ‘Rate’ refers to the rate of the reaction and k is the rate constant of the reaction.
This differential form can be rearranged and integrated on both sides to get the required Integral form as shown below.
⇒Rate = dt −dA=k, multiplying on both sides with dt we get;
⇒d[A]=−kdt, integrating on both sides we get,
[A]0∫[A]d[A]=−0∫tkdt
Where [A]0 is the initial concentration of the reactant [A] at time t=0. Solving for [A], we get:
⇒[A]=[A]0kt
⇒[A]=−kt+[A]0
Which is the required integral form. On comparing this equation with the equation of a straight line (y=mx+b), an [A] against t graph can be plotted to get a straight line with slope equal to −k and intercept equal to [A]0 as shown below.
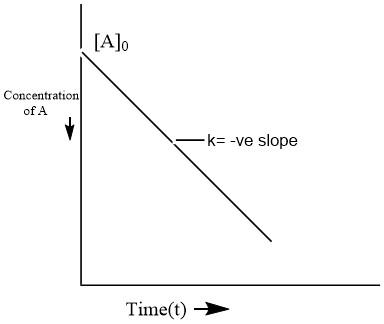
Here, we can see that the graph of a zero-order reaction is linear with -ve slope and non-zero intercept.
So, the correct answer is Option C.
Note: Other than zero-order reaction, there are first order reaction and second order reaction, whose graphs are different from zero-order reaction. The reaction of hydrogen with chlorine (Photochemical reaction) and decomposition of nitrous oxide over a hot platinum surface are examples of zero-order reaction.
