Question
Question: For a reaction, $A \rightleftharpoons P$, the plots of [A] and [P] with time at temperatures $T_1$ a...
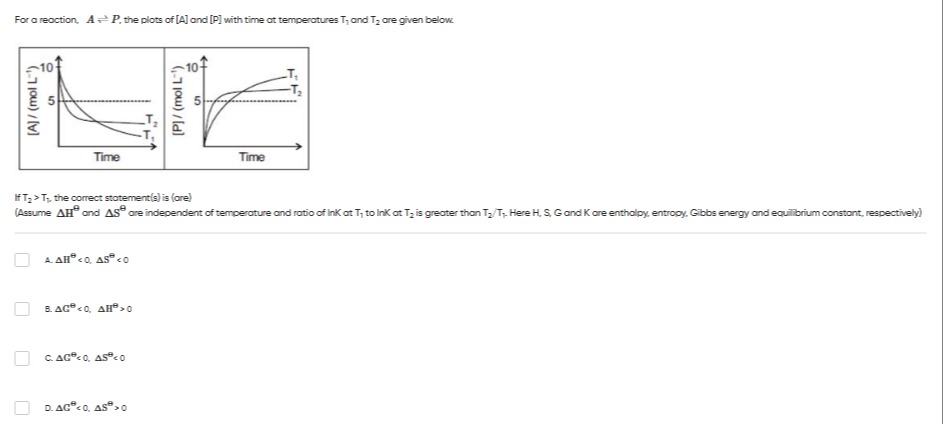
For a reaction, A⇌P, the plots of [A] and [P] with time at temperatures T1 and T2 are given below.
If T2>T1, the correct statement(s) is (are) (Assume ΔH⊖ and ΔS⊖ are independent of temperature and ratio of lnK at T1 to lnK at T2 is greater than T2/T1. Here H, S, G and K are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively)
ΔH⊖<0, ΔS⊖<0
ΔG⊖<0, ΔH⊖>0
ΔG⊖<0, ΔS⊖<0
ΔG⊖<0, ΔS⊖>0
ΔG⊖<0, ΔS⊖>0
Solution
At T1, [A]eq>[P]eq, so K1<1. At T2, [A]eq<[P]eq, so K2>1. Since T2>T1 and K increases with temperature, the forward reaction is endothermic (ΔH⊖>0). Since K2>1, the reaction is spontaneous at T2, so ΔG⊖(T2)<0. Using ΔG⊖=ΔH⊖−TΔS⊖, we have ΔG⊖<0 and ΔH⊖>0. Thus, ΔH⊖−TΔS⊖<0, which implies ΔH⊖<TΔS⊖. Since ΔH⊖>0, for this to hold, ΔS⊖ must be positive (ΔS⊖>0). Therefore, ΔG⊖<0 and ΔS⊖>0.
