Question
Question: For a reaction, \(A \rightleftharpoons P\), the plots of \(\left[ A \right]\) and \(\left[ P \right]...
For a reaction, A⇌P, the plots of [A] and [P] with time at temperature T1 and T2 are given below. If T2>T1, the correct statement(s) is/are:
(Assume ΔHθ and ΔSθ are independent of temperature and ratio of lnK at T1 and lnK at T2 is greater than T1T2. Here, H,S,G and K are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.)
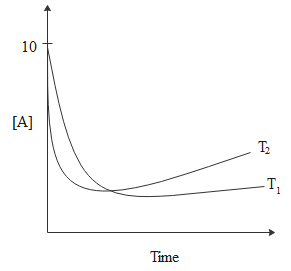
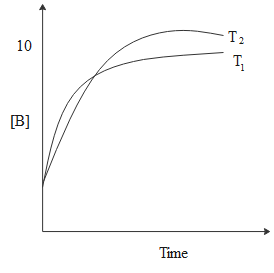
A.ΔHθ<0,ΔSθ<0
B.ΔHθ>0,ΔGθ<0
C.ΔSθ<0,ΔGθ<0
D.ΔSθ>0,ΔGθ<0
Solution
To solve this question, you must recall the relation between the Gibbs free energy and equilibrium constant of a reaction. Gibbs free energy is a thermodynamic potential that is used to calculate the maximum work that can be performed by a system at a constant pressure and temperature.
Formula used:
ΔGθ=−RTlnKeq
Where, ΔGθ is the change in free energy of reaction, Keq is the equilibrium constant of the reaction, R is the gas constant and T is the temperature.
Complete step by step answer:
In the given reaction,A⇌P
From the graph, we can see that at equilibrium [A]<5 and [P]>5.
Thus, we can write the equilibrium constant as, Keq=[P][A]>1.
Since ΔGθ=−RTlnKeq, we can say that,ΔGθ<0.
We are given in the question that T2>T1.
From this we can infer, that,lnKT2lnKT1>T1T2>1
⇒KT2KT1>1 ⇒KT2<KT1
This implies that the given reaction is exothermic which means that the change in enthalpy of the reaction will be zero.
ΔHθ<0
Also, we know that,
ΔGθ=ΔHθ−TΔSθ ⇒ΔSθ=TΔHθ−ΔGθ
Since ΔHθ<0 and ΔGθ<0, it is necessary for the change in entropy to be negative; ΔSθ<0.
Therefore, the correct options are A and C.
Note:
The importance of the Gibbs function is that it is the single master variable that can determine whether a certain chemical change is thermodynamically possible. If the free energy of the reactants is greater than that of the products the reaction takes place spontaneously. ΔGθ is a key quantity in determining whether a reaction will take place in a given direction or not. For most reactions taking place in solutions or gaseous mixtures, the value of ΔGθ depends on the proportions of the various reaction components in the mixture; it is not a simple sum of the "products minus reactants" type, as is the case with ΔHθ.
