Question
Question: For a linear plot of \[\log (x/m)\] versus \[\log p\] in a Freundlich adsorption isotherm, which of ...
For a linear plot of log(x/m) versus logp in a Freundlich adsorption isotherm, which of the following statements is correct ? (kand n are constants)
A. n1 appears as the intercept.
B. Only n1 appears as the slope.
C. logn1 appears as the intercept.
D. Both k and n1 appear in the slope term
Solution
An adsorption isotherm is a curve relating the equilibrium concentration of a solute on the surface of an adsorbent. The adsorption isotherm is also an equation relating the amount of solute adsorbed onto the solid.
Complete step by step answer:
Freundlich Adsorption Isotherm gives the variation in the quantity of gas adsorbed by a unit mass of solid adsorbent with the change in pressure of the system for a given temperature. The expression for the Freundlich isotherm can be represented by the following equation;
⇒mx=kPn1
Where x is the mass of the gas adsorbed, mis the mass of the adsorbent, P is the pressure and n is a constant which depends upon the nature of adsorbent and the gas at a given temperature. Taking the logarithm on both the sides of the equation, we get;
⇒logmx=logk+n1logP
On comparing this equation with the equation of straight line(y=mx+c). The plot of this equation is a straight line as represented by the following curve.
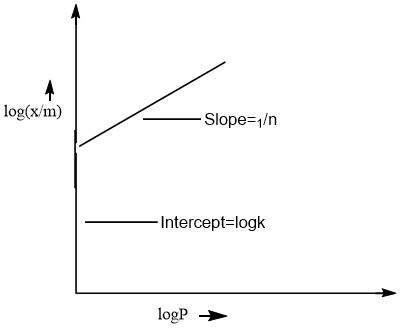
From the graph, it is very clear that only 1/n appears as the slope.
So, the correct answer is Option B.
Note: The Freundlich adsorption isotherm is followed by another two isotherms, Langmuir adsorption isotherms and BET theory. The Langmuir adsorption isotherms predict linear adsorption at low adsorption densities and a maximum surface coverage at higher solute metal concentrations.
