Question
Question: For a hydrogenic quantum system, the probability of finding the electron $D_{n,l}(r)dr$ in a spheric...
For a hydrogenic quantum system, the probability of finding the electron Dn,l(r)dr in a spherical shell of radius r (with nucleus at its center) and thickness dr, is plotted against r (in units of a0) for three sets of values of n and l (plots I, II, and III). The principal quantum number (n), azimuthal quantum number (l) and area under the curve (A) corresponding to the three plots are denoted by subscripts I, II, and III respectively. (The plots are not drawn to scale.)
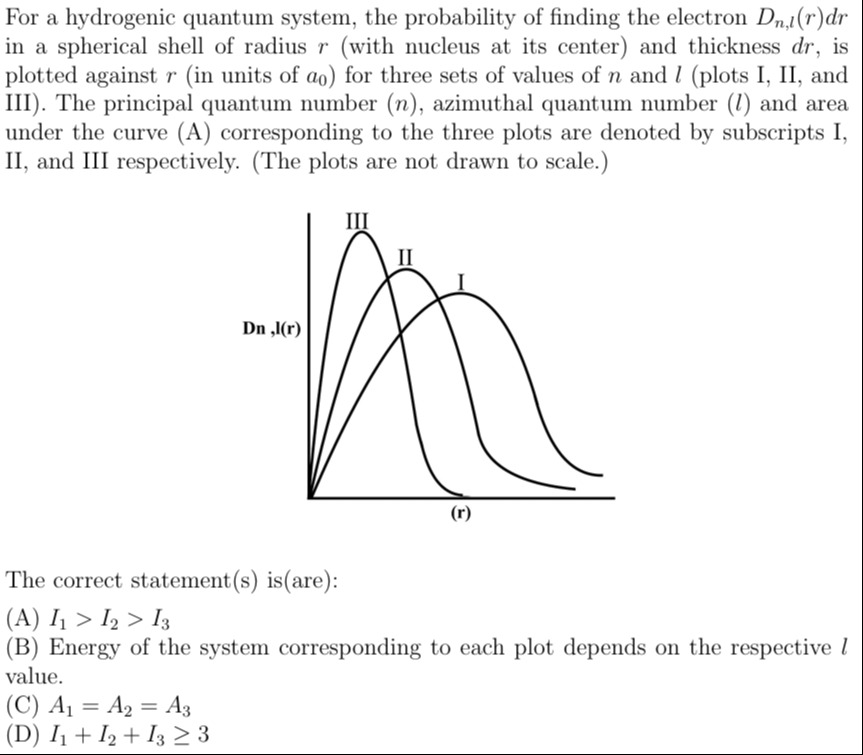
I1>I2>I3
Energy of the system corresponding to each plot depends on the respective l value.
A1=A2=A3
I1+I2+I3≥3
A, C, D
Solution
The problem describes the radial probability distribution Dn,l(r) for a hydrogenic quantum system. We are given three plots (I, II, III) showing Dn,l(r) versus r. We need to identify the correct statement(s) regarding the principal quantum number (n), azimuthal quantum number (l), and the area under the curve (A) for these plots.
Understanding the Radial Probability Distribution Dn,l(r):
Dn,l(r)dr is the probability of finding the electron in a spherical shell of radius r and thickness dr. The function Dn,l(r) is given by 4πr2Rn,l(r)2, where Rn,l(r) is the radial part of the wave function.
Analyzing the Plots:
-
Number of Peaks/Nodes: Each plot (I, II, III) shows a single peak and starts from zero at r=0. This shape is characteristic of orbitals with no radial nodes. The number of radial nodes is given by n−l−1. If there are no radial nodes, then n−l−1=0, which means l=n−1. Examples of such orbitals are 1s (n=1,l=0), 2p (n=2,l=1), 3d (n=3,l=2), 4f (n=4,l=3), etc.
-
Position of Peaks: From the graph, the most probable radius (the position of the peak) for the three plots follows the order: rpeak,I>rpeak,II>rpeak,III. For hydrogenic atoms, the most probable radius for orbitals with l=n−1 is approximately proportional to n2. Specifically, rmp=Zn2a0. Therefore, nI2>nII2>nIII2, which implies nI>nII>nIII.
Evaluating the Options:
(A) I1>I2>I3
The problem states that n,l,A for the three plots are denoted by subscripts I, II, III. So, I1,I2,I3 in the options refer to nI,nII,nIII respectively. Based on our analysis of the peak positions and the characteristic of orbitals with no radial nodes, we concluded that nI>nII>nIII. Thus, statement (A) is correct.
(B) Energy of the system corresponding to each plot depends on the respective l value.
For a hydrogenic quantum system (like H, He+, Li2+), the energy of an electron in a given orbital depends only on the principal quantum number n. The formula for energy is En=−n2Z2RH, where RH is the Rydberg constant. The energy is independent of the azimuthal quantum number l. Thus, statement (B) is incorrect.
(C) A1=A2=A3
The area under the radial probability distribution curve Dn,l(r) from r=0 to r=∞ represents the total probability of finding the electron in all of space. Since the electron must be found somewhere, this total probability must be equal to 1 for any normalized wave function. ∫0∞Dn,l(r)dr=1 Therefore, the area under each plot (AI,AII,AIII) must be equal to 1. Thus, AI=AII=AIII. Statement (C) is correct.
(D) I1+I2+I3≥3
Assuming I1,I2,I3 refer to nI,nII,nIII respectively. From the analysis of (A), we established nI>nII>nIII. Since n is a principal quantum number, its minimum value is 1. For distinct n values following the order nI>nII>nIII, the smallest possible integer values are nIII=1, nII=2, and nI=3. In this case, nI+nII+nIII=3+2+1=6. Since 6≥3, the statement nI+nII+nIII≥3 is correct.
Based on the analysis, statements (A), (C), and (D) are correct.
