Question
Question: For a first order reaction, if \[{K_1}:{K_2}:{K_3}\] is 1:2:3, then which of the subsequent statemen...
For a first order reaction, if K1:K2:K3 is 1:2:3, then which of the subsequent statement(s) is/are incorrect? ([A]0 = 1 M)
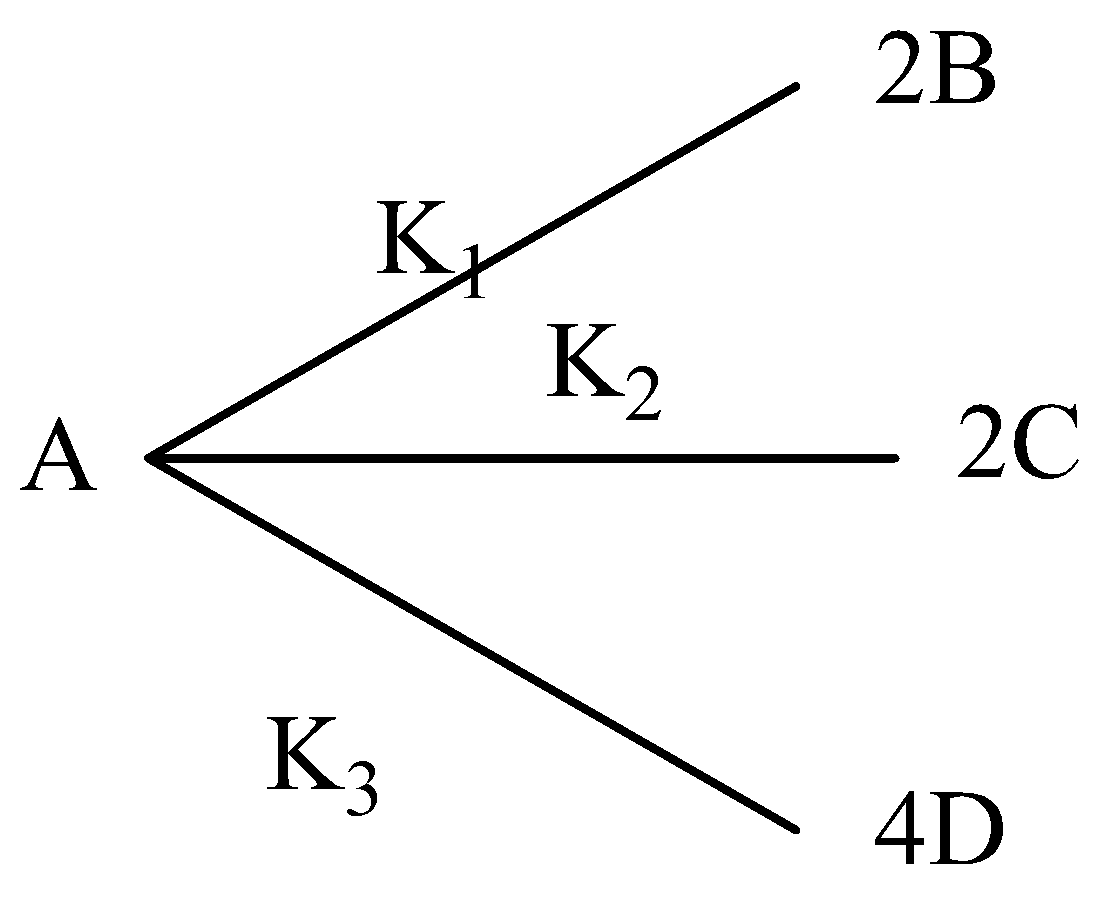
A.At time t∞,[C] is 2/3 M
B.[B]t⩾[C]t
C.When [A]t = ½ M, then [D] = 1M
D.[A]t+2[B]t+2[C]t+2[D]t=1M
Solution
We have to know that for a first order reaction, rate of reaction is directly proportional to concentration of one reactant. Once we discover the value of reaction constant (K), we will find the half-life period of a first order reaction.
Complete answer:
As we know that in these reactions the rate of reaction depends on the concentration of one reactant only. There are often many reactants within the reaction but concentration of just one reactant will affect the rate of reaction. Concentration of other reactants will haven’t any effect on order of reaction.
From the question;
Option A: The initial concentration of A is 1 M out of this 0.33M will react to sort 2/3 at B. Another 0.33M of A will react to sort at 2/3 C. The remaining 0.33M A will react to sort 4/3 at D.
Hence, at infinite time, the concentration of C is 2/3 M. this feature is correct.
Option B: This is often because the value of the equilibrium constant K2 is greater than that of K1 as K1:K2 = 1:2. Hence more C will be formed than B. Thus [B]t < [C]t . So this feature is wrong.
Option C: When [A]t=1/2M the concentration of A that has reacted is 1/2M. Out of this, 1/4M (A) has reacted to sort 1M at D. So this feature is correct.
Option D: The initial concentration of A is 1M. Each molecule of A gives two molecules of B or two molecules of C or four molecules of D. Thus when the total number of molecules of A that are unreacted and that are reacted to sort B, C and D are added, they will be the same as an initial number of molecules of A.
[A]t represents the molar concentration of A that is unreacted .2[B]t represents the molar concentration of A that has reacted to form B.
2[C]t represents the molar concentration of A that has reacted to sort C. 2[D]t represents the molar concentration of A that has reacted to sort D.
Thus [A]t+2[B]t+2[C]t+4[D]t=1M . So this feature is also correct.
Therefore, the solution to this present question is option B.
Note:
We must remember that the equations of rate of reaction and half-life period are different for reactions of different order. If order of reaction isn’t given in the question check the units of constant (k) because the units of constant (k) are different for different order reactions.
