Question
Question: For a chemical reaction variation in concentration, In \(R\) Vs time (s) plot is shown below. (i)...
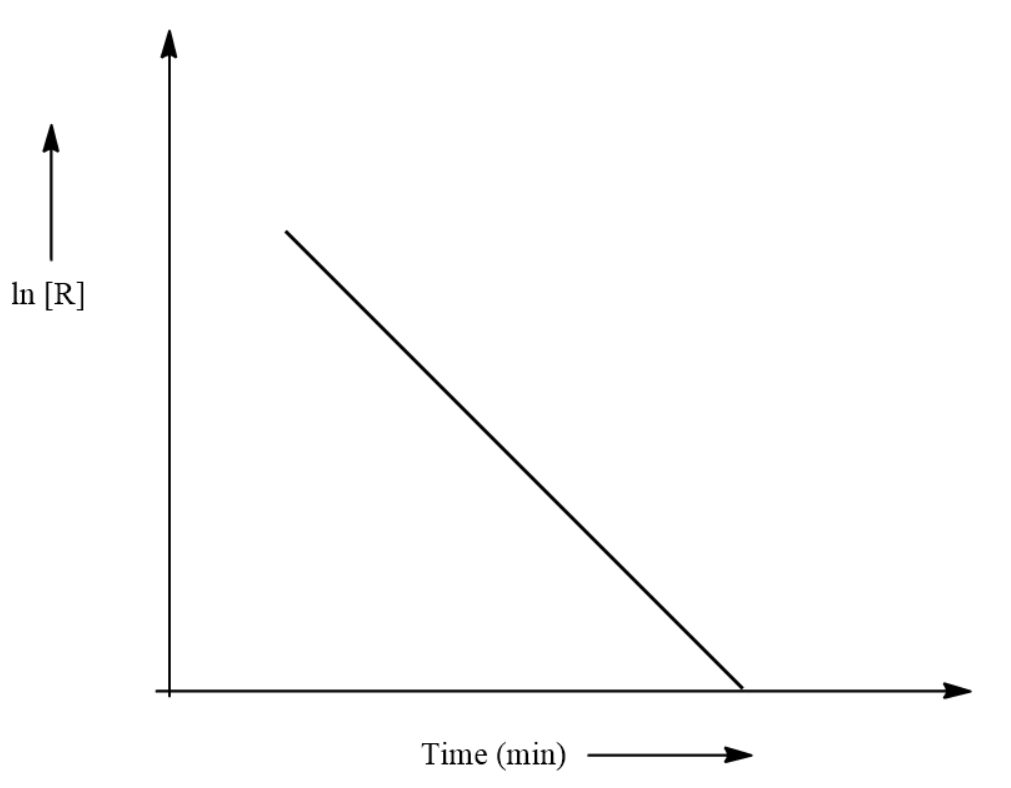
For a chemical reaction variation in concentration, In R Vs time (s) plot is shown below.
(i) What is the order of the reaction?
(ii) What are the units of rate constant (k)?
(iii) If the initial concentration is half of the original concentration, how will t1/2 change?
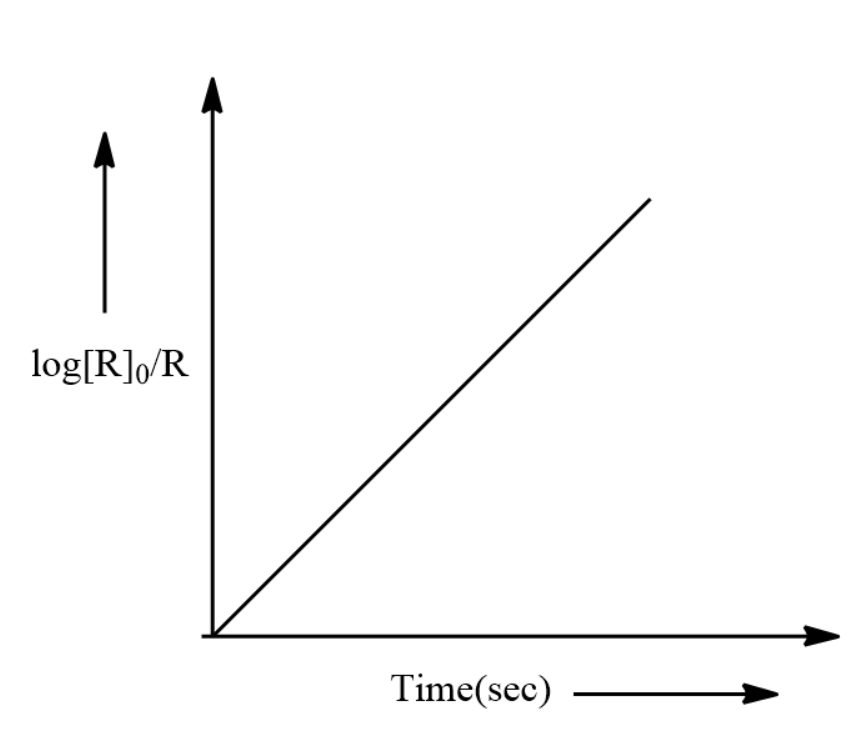
(iv) Draw the plot of RlogR0 vs time.
Solution
We need to know what each of these terms means to answer this question. The Order of Reaction refers to the power of the rate on the concentration of each reactant in the rate law expression. t1/2 is the half-life of the reaction which is the amount of time taken for the reactant to become half of its initial concentration during the reaction.
Complete answer:
(i) Let us consider that the slope of the graph is –k where k is a constant.
We can say that the equation of this graph will be
⇒tlog[R]=−k
When we substitute for t as 0 and R as the initial concentration we get:
⇒R=R0e−kt
This is the equation for a first order reaction
(ii) We know that the unit for rate constant of a first order reaction is sec−1
(iii) For a first order reaction the formula for t1/2 is given by:
⇒t1/2=k0.693
Where k is the rate constant.
As we can see there is no dependence of concentration term on the half-life we can say that there is no effect on t1/2 when the initial concentration is half of the original concentration.
(iv)
Note:
To answer questions using graphs, always frame the equation of the given graph. In this example since it is a straight line we used the formula y=mx.
To find the unit of rate constant of any order, we can use the following formula for easiness:
⇒mol(1−n)L(n−1)s−1
Where n is the order of the reaction.
