Question
Question: For a chemical reaction \( R \to P \) , the variation in the concentration \( \left( R \right) \) vs...
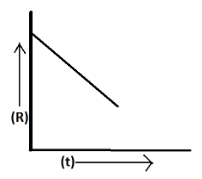
For a chemical reaction R→P , the variation in the concentration (R) vs. time (t) plot of the solution is given as above
(a) predict the order of reaction
(b) What is the slope of the curve?
Solution
Hint : The order of reaction is defined as the power dependence of rate on the concentration of all the reactants present. For example, the rate of a first order reaction depends on the concentration of one of the species in the reaction. The slope of the curve is found using y=mx+c .
Complete Step By Step Answer:
The concentration of the given reactant varies linearly with time. The initial concentration of reactant [R] is given by [Ao] at zero degrees from the given graph hence it is a zero order reaction. For first order reaction the graph is parabolic. The equation is given by the integrated rate law equation; [A]=[Ao]−kt .
Now, to find slope from the given equation above;
Slope =−k
Slope of equation is given by;
y=mx+c
From the above equation m=−k
So the answers of the given question is,
The given reaction is first order reaction
And the slope of the graph is m=−k
Additional Information:
The characteristics of the reaction order are given below;
The reaction order represents the number of species whose concentration affects the rate of reaction
We get this by adding all the exponents of the concentration terms in the given rate expression
The order does not depend on stoichiometric coefficient corresponding to each species in the given balanced equation
It is always defined with the help of reactant concentrations not the product
The value of this zero order reaction can be in the form of an integer. It can also have a value of zero.
The expression of rate law is given by; r=k[A]x[B]y
Note :
The rate of first order reaction depends on the concentration of one reactant hence it is said to be first order reaction. In this reaction there is presence of many reactants but only one will be of first order concentration.
