Question
Question: For \(4\) -pentanoic acid when treated with \({I_2}\) and \(NaHC{O_3}\) gives: A. \(4,5-diiodo pen...
For 4 -pentanoic acid when treated with I2 and NaHCO3 gives:
A. 4,5−diiodopentanoicacid
B. 5−iodomethyl−dihydrofuran−2−one
C. 5−iodotetrahydropyran−2−one
D. 4−pentenoyliodide
Solution
To solve this question try to recall Iodolactonization. Halolactonization is a reaction in which a ring (lactone) is formed by the addition of oxygen and iodine to the side of the carbon-carbon double bond. Halolactonization generally forms four to six-membered lactones. The strength of the reaction comprises the mild conditions of the versatile iodine atom into the product.
Complete answer:
When 4−pentanoicacid when treated with I2 and NaHCO3 , there is a formation of a positively charged Iodonium ion in the molecule that contains a carboxylic acid. The oxygen of the carboxyl acts as a nucleophile and attacks the Iodonium ion and forms a lactone ring. 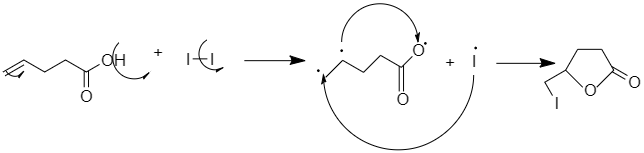
The reaction is performed under the basic condition to increase the nucleophilicity of the carboxyl group.
So the correct answer is option B.
4−pentenoicacid when treated with I2 and NaHCO3 gives 5−iodomethyl−dihydrofuran−2−one .
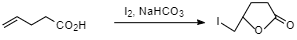
Additional Information: “lactone” name was coined by a French chemist Theophile-Jules Pelouze. The word lactone derives from the ring compound called lactide, formed from dehydration of $2-hydroxypropanoic acid. Lactones contribute to the flavor of fruit, unfermented and fermented dairy products.
Note:
Stereoselective Iodolactonization is used to synthesize many natural products including the medicinal application such as vernolepin and venomenon, used in tumor inhibition. Vibralactone is a pancreatic lipase inhibitor that is used in the treatment of obesity. Iodolactonization is also used to synthesize prostaglandins.
Bromolactonization is much less used because the electrophilic addition of bromine to alkene can compete with bromolactonization reaction and reduces the yield of the desired lactone.
