Question
Question: For \(3 - {{methyl}} - 2 - {{butanol}} \) on treatment with \(\xrightarrow {{dryHCl}} \)gives predom...
For 3−methyl−2−butanol on treatment with dryHClgives predominantly:
A.2−chloro2−methylbutane
B.2−chloro2−methylbutane
C.2− 2−dimethylbutane
D.None of the above.
Solution
To attempt this question first draw the structure of 3−methyl\ [- 2 - ]butanol and treat it with hydrochloric acid orHCl. The product will be a carbocation, after getting the product we have to look for the stability of carbocation and rearrange it accordingly.
Complete step by step answer:
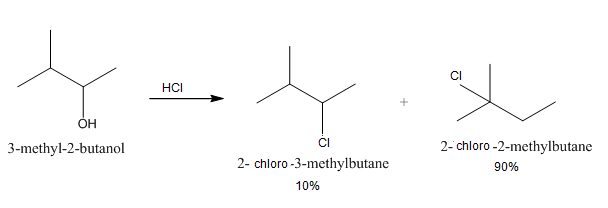
The mechanism of this reaction involves 4 steps
Step: 1
Protonation: is also known as hydro nation is addition of proton or a hydrogen cation to an atom, molecule. This forms a conjugate acid of that particular compound. In this question the 3−Methyl\ [- 2 - ]butanol accepts a pair of protons which gets attached to the ′OH′ ion.
Step: 2
Now the formation of carbocation takes place. Carbocation is a molecule in which a carbon atom has 3 positive charges. This is also known as carbonium ion. It is divided into 3 groups, primary, secondary and tertiary. After the attachment of the proton to the ′OH′ ion a secondary carbocation is formed by the elimination of water H2O.
Step: 4
After the elimination of water, a hydride shift takes place. Hydride shift is a type of rearrangement in a carbocation. In this type of shift the hydrogen atom in a carbocation migrates to the carbon atom which has the formal charge of +1(carbon2) from an adjacent carbon (carbon1).
Step: 5
After the hydride shift the tertiary carbocation is formed which is more stable. Now the ′Cl′ molecule attacks on the tertiary carbocation which is also known as the nucleophilic attack.
The answer is A. 2− 2−chloro2−methylbutane
Note:
Unimolecular substitution reactions are favoured when tertiary substrates such as 3-methyl2-butanol are reacted with a good nucleophile such as chlorine ion under protic aprotic conditions that is in acidic medium.
