Question
Question: $F^{\ominus}$ $Cl^{\ominus}$ $Br^{\ominus}$ $I^{\ominus}$ Stability?...
F⊖ Cl⊖ Br⊖ I⊖
Stability?
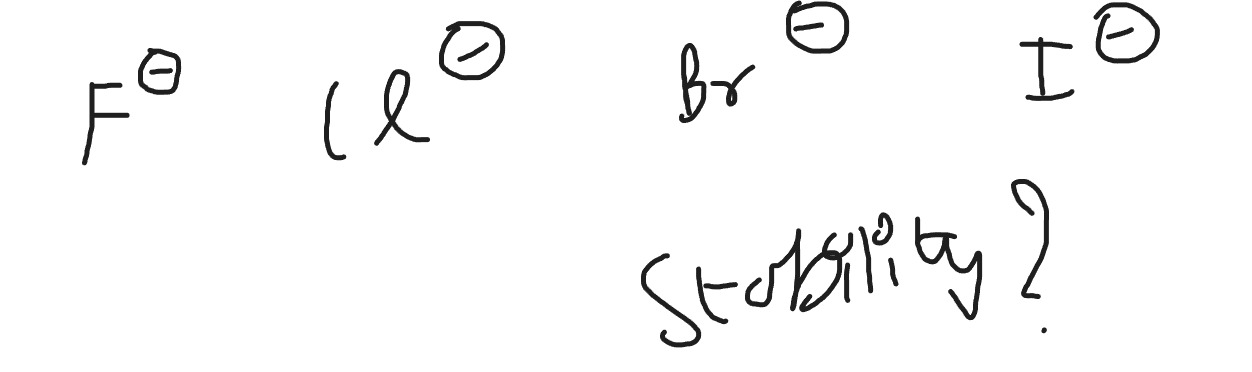
Answer
F⁻ < Cl⁻ < Br⁻ < I⁻
Explanation
Solution
The stability of halide ions increases with ionic size. A small ion like F⁻ has a high charge density, leading to greater electron-electron repulsion, thus it is less stable. In contrast, as we move down the group to I⁻, the larger size allows the negative charge to be more delocalized, reducing repulsion and increasing stability. Therefore, the order of stability is:
F−<Cl−<Br−<I−