Question
Question: Following is an example of an \(E2\) reaction 
A.True
B.False
Solution
We know the elimination reaction is a kind of organic reaction where two substituents are removed from a molecule in a one- or two-step process. If the mechanism proceeds in one-step, then it is called as E2 reaction and if the reaction proceeds in two-step, then it is called as E1 reaction. E2 reaction is bimolecular reaction, whereas E1 reaction is unimolecular reaction.
Complete step by step solution:
We have to remember that an elimination reaction is the class of reactions mostly used for the conversion of saturated compounds to unsaturated compounds. The action of acids, bases, or metals results in the elimination of several groups of atoms or pairs of atoms from a molecule. It can also occur by the process of heating at high temperature.
We know that the E2 reaction is a bimolecular reaction and takes place in one step. In E2 elimination reaction the carbon-hydrogen and carbon-halogen bonds are broken, and a double bond is formed. In E2 reaction, a strong base is used such that it removes weakly acidic hydrogen.
We know that the E1 reaction is a unimolecular reaction and takes place in two steps. The two major steps in the E1 reaction are ionization and deprotonation. In the ionization step, the carbon-halogen bond breaks and intermediate carbocation is formed. In the second step, carbocation is deprotonated.
The given reaction is,
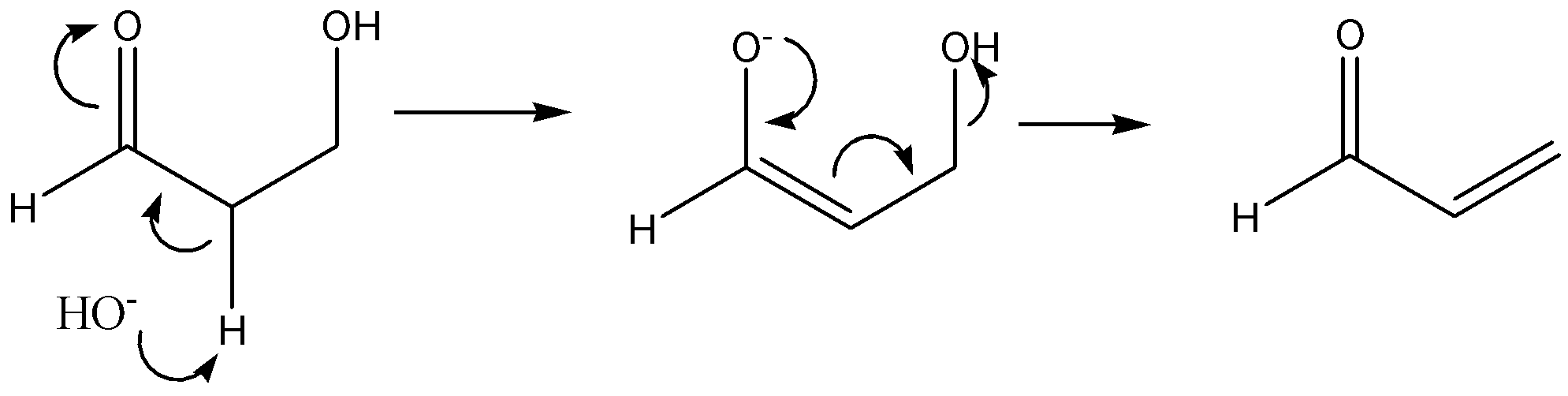
The above reaction is an acid-catalyzed dehydration of enol. The acid-catalysed dehydration of enol is an example of elimination reaction. It involves the removal of molecules of water (or) in the removal of a molecule of alcohol. In this reaction, HOH substituent is removed that leads to the formation of a double bond. Thus, this reaction is an example of an E1 elimination reaction.
Therefore,option (B) is correct.
Note:
We have to remember that an E1 mechanism shares the characteristics of the SN1 reaction. The 1st step in the E1 reaction is the formation of carbocation as an intermediate by the removal of the leaving group. Generally, this step takes a long time to complete and this is the rate-determining step.
