Question
Question: Following are the transition metal ions of 3d series: \({\text{T}}{{\text{i}}^{{\text{4 + }}}}{\te...
Following are the transition metal ions of 3d series:
Ti4 + ,V2 + ,Mn3 + ,Cr3 +
(Atomic number: Ti=22, V= 23, Cr-24,Mn=25)
Answer the following:
(i) Which ion is most stable in an aqueous solution and why?
(ii) Which ion is a strong oxidizing agent and why?
(iii) Which ion is colourless and why?
Solution
Most stable ion is an ion that has a full-filled or half-filled electronic configuration. The species having high reduction potential will be the strongest oxidizing agent. The ion having no electron in orbitals will be colourless.
Complete step by step answer:
(i) We can determine the most stable ion in an aqueous solution as follows:
Each metal reacts with water ligands and forms an octahedral complex as follows:
Mn + + 6H2O→[M(H2O)6]n +
As the water ligand approaches metal, the d-orbitals of metal split into two parts, three orbitals of lower energy designated as t2g and two orbitals of higher energy designated as eg.
The valence electrons of the metal get rearranged in these two set of orbitals. The water is a weak field ligand, so it does not cause pairing. The full-filled and half-filled electronic configuration are most stable thus stabilizing the metal ion.
The valence electronic configuration of each metal ion, its crystal field splitting and filling of electrons is shown as follows:
Valence electronic configuration of Ti4+=3d0
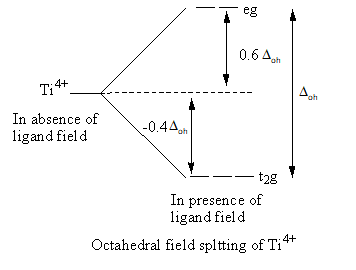
So, the electronic configuration of the titanium metal on forming the complex is t2g0 .
Valence electronic configuration of V2+=3d3
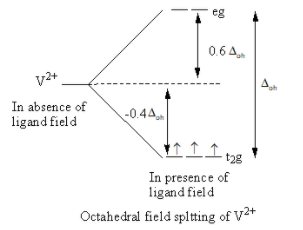
So, the electronic configuration of the vanadium metal on forming the complex is t2g3 .
Valence electronic configuration of Cr3+=3d3
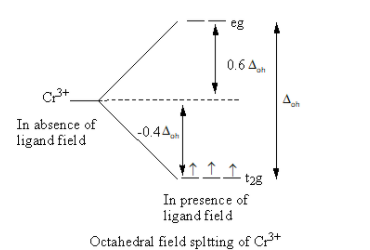
So, the electronic configuration of the chromium metal on forming the complex is t2g3 .
Valence electronic configuration of Mn3+=3d4
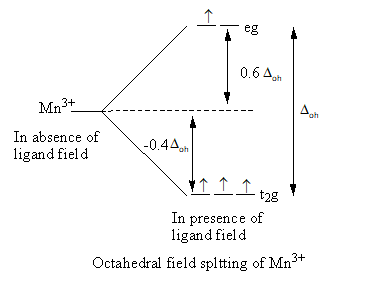
So, the electronic configuration of the manganese metal on forming the complex is t2g3eg1 .
So, vanadium V2 + and chromium metal ionCr3 + have stable half-filled electronic configuration Ti4 + ,V2 + ,Mn3 + ,Cr3 +
Therefore, vanadium V2 + and chromium metal ion Cr3 + are most stable in an aqueous solution due to stable half-filled electronic configuration.
(ii) Determine the strongest oxidizing agent as follows:
The reduction potential of Mn3 + /Mn2 + is highest 4.45V , V3 + /V2 + is 0.26V, Cr3 + /Cr2 + is −0.41V and Ti4 + /Ti3 + is highest 0.1V.
So, the reduction potential of Mn3 + is highest so, will reduced easily and cause oxidation of another species so, it is the strongest oxidising agent among all four.
Therefore, Mn3 + ion is a strong oxidizing agent due to high reduction potential.
(iii) Determine the colourless ion as follows:
The colour is produced by d-d transition means electron from one d-orbitals goes into another d-orbitals by releasing energy. The energy lies in the visible region, so a colour is observed. The metal ion Ti4 + has no electron in orbitals so, d-d transition cannot take place so, the Ti4 + is colourless.
Therefore, Ti4 + ion is colourless due to absence of electrons in the metal orbitals in complex.
Note: The t2g3 is a more stable configuration then t2g3eg1. The presence of one electron in eg causes the John Teller distortion. High the reduction potential, stronger will be the oxidizing agent. The d-d transitions are responsible for colour in transition metals.
