Question
Question: Fluorobenzene (\[{C_6}{H_5}F\]) can be synthesised in the lab: A.By heating phenol with \[HF\]and ...
Fluorobenzene (C6H5F) can be synthesised in the lab:
A.By heating phenol with HFand KF
B.From aniline by diazotization followed by heating the diazonium salt with HBF4
C.By direct fluorination of benzene with F2gas
D.By reacting bromo benzene with NaFsolution.
Solution
First we have to discuss the structure of Fluorobenzene. Therefore, the name suggest it’s a molecule with Fluorine attached to a benzene having a chemical formula of C6H5F.The process of synthesising of fluorobenzene contains the process of diazotization.
Complete step by step answer:
The structure of Fluorobenzene as follow:
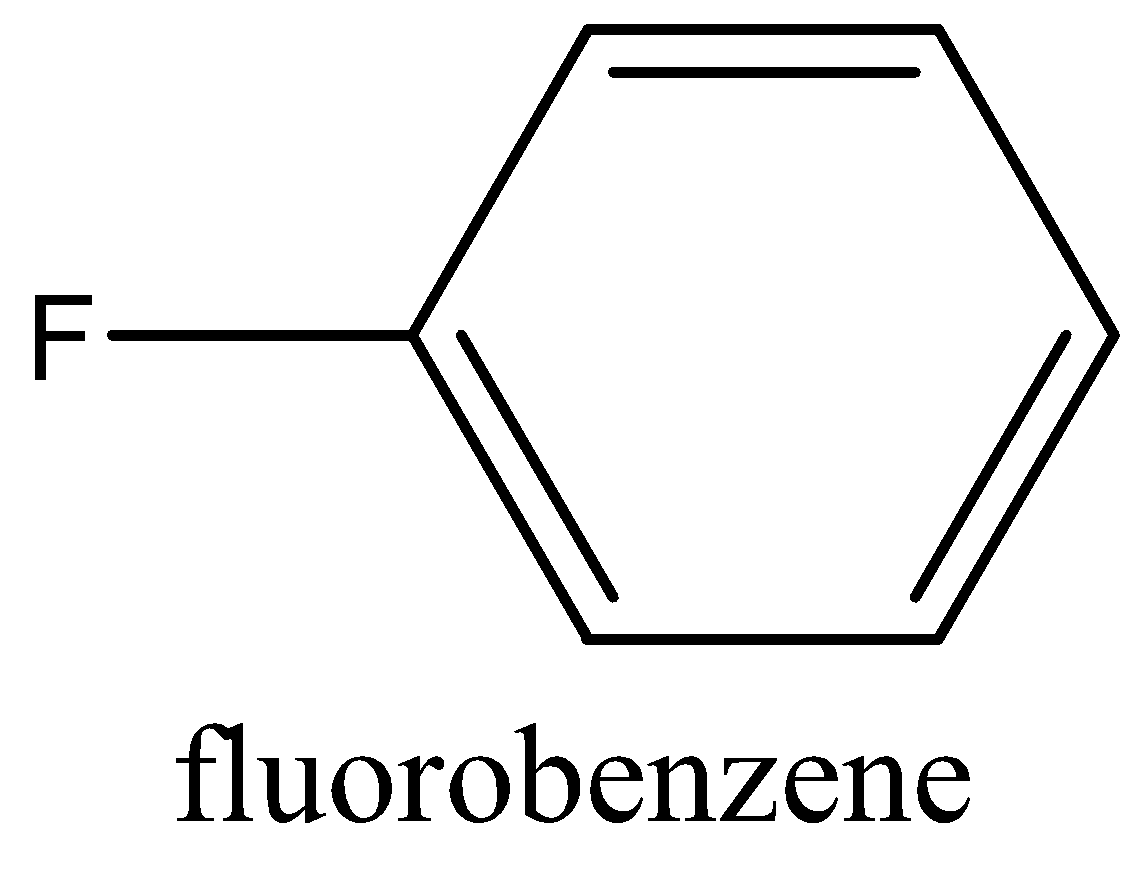
Now coming to the question we are asked how fluorobenzene can be synthesized. To synthesis fluorobenzene we first need to have aniline. The aniline is then converted into a diazonium salt with the process known as diazotization. In diazotization the aromatic amine like aniline is converted to a diazonium salt. The diazonium salt is having a chemical formula of R−N2Cl, R is a benzene ring. After getting diazonium salt we heat it with HBF4to get the required product that is fluorobenzene. The reaction is given below:
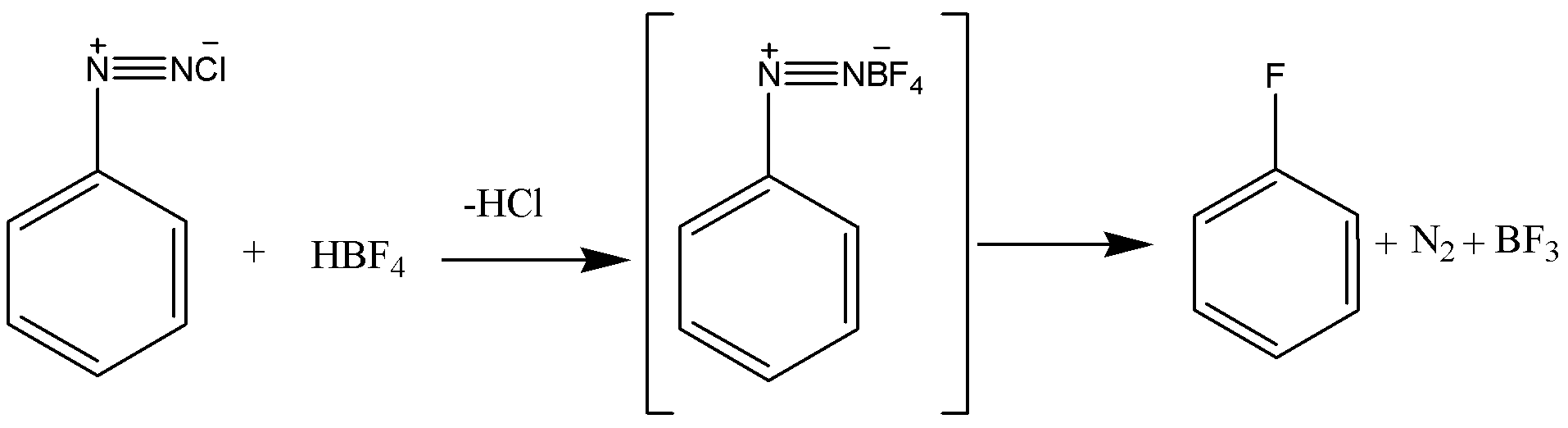
Hence the answer to this question is option B. From aniline by diazotization followed by heating the diazonium salt with HBF4
Note:
We can use Fluorobenzene as a solvent for highly reactive species. It is to be noted that this molecule's melting point is even below that of benzene. The melting point of Fluorobenzene is −44oC. Also the difference in boiling point between the two elements is only 4oC. This molecule is more polar than that .
