Question
Question: Fluorine shows only +1, -1 oxidation state while other halogen elements show +3, +5 and +7 oxidation...
Fluorine shows only +1, -1 oxidation state while other halogen elements show +3, +5 and +7 oxidation states in addition to +1, -1, why?
Solution
Hint: Before attempting this question, prior knowledge of finding out electronic configuration, electrostatic force, and oxidation states is important.
Complete step by step solution:
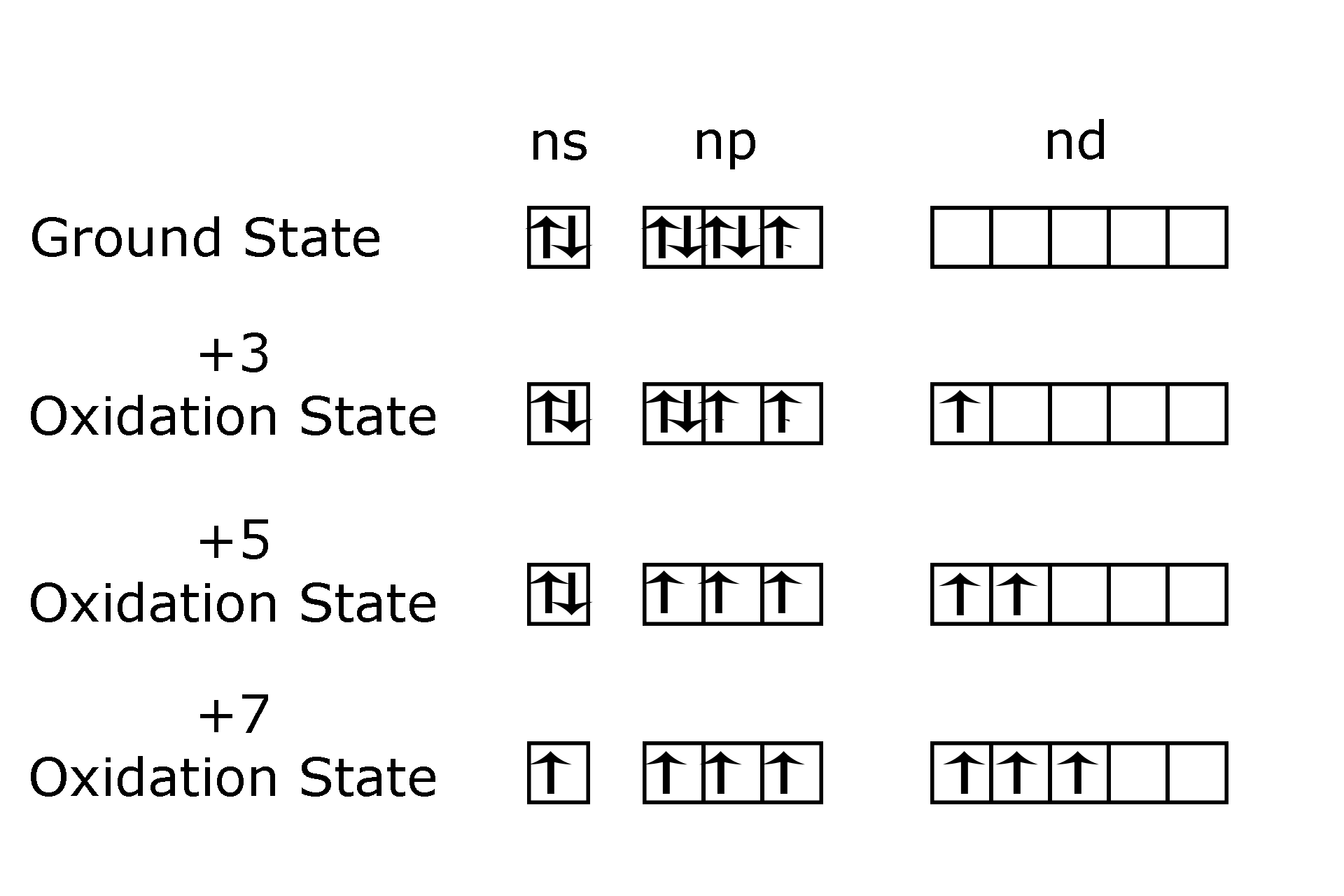
Let’s start with discussing what oxidations states halides can acquire. The general electronic configuration of halides is1s22s22p63s23p5. As can be seen in the general electronic configuration, there are 7 electrons in the outermost shell of halides that can be used in bonding. The inner electrons do not take part in bonding, as there is a very strong electrostatic force of attraction between the nucleus and the electrons.
So in halides, there are two possibilities, they can either gain 1 electron to achieve stable configuration, or, they can donate till 7 electrons to achieve stable configuration .That’s how they can have +1,-1, +3, +5 and +7 oxidation states.
A point to note is, with the donation of each electron, it becomes more and more difficult to donate the next electron. So +7 configuration is rarer than +5 and so on.
Halides like Cl, Br and I can achieve +1,-1, +3, +5, and +7 due to the presence of d orbital, which are utilized in the following manner-
Some examples of such compounds is:
- +1 in NaClO
- +3 in NaClO2
- +5 in NaClO3
- +7 in NaClO3
- -1 in HCl
But, the case of F is a little different, it shows only +1, and -1, because it does have a d orbital which can accommodate electrons as shown above.
Note: Fluorine is a very reactive element, rarely found in elemental state in nature. The uses of Fluorine are very limited in industrial culture, in comparison to other halides like Chlorine (Cl) (found in table salt, chloroform), Iodine (I) (used in starch test), etc.