Question
Question: Fluorine molecules are formed by A) The axial p-p orbital overlap B) The sideways p-p orbital ov...
Fluorine molecules are formed by
A) The axial p-p orbital overlap
B) The sideways p-p orbital overlap
C) The s-s orbital overlap
D) The s-p orbital overlap
Solution
Fluorine molecule is bonded by strong sigma bond. This type of covalent bond is formed by the head-to-head overlap of bonding orbitals along the internuclear axis. This is known as axial overlap or head-on overlap. p-p overlapping takes place when two half-filled p-orbitals of the two atoms approach each other.
Complete answer:
Before going to explain this solution. We will go with each option.
(a) The axial p-p orbital overlap:
In fluorine 2pz orbitals are partially filled they will overlap in head to head fashion. Hence strong sigma bonds will be formed. Fluorine electronic configuration is 1s22s22p5 . So the last electron will go in pz orbital.
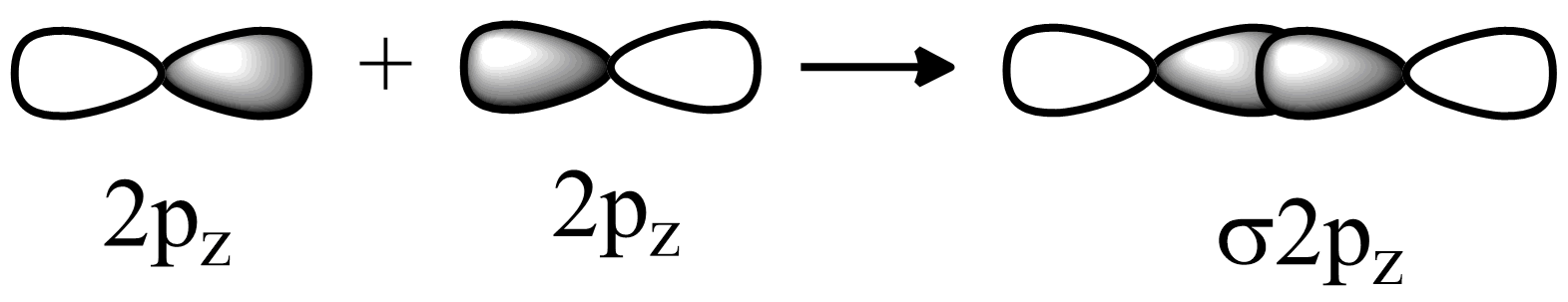
(b) The sideways p-p orbital overlap-
This type of overlapping is possible in px and py orbital. These orbits undergo sideways overlapping and the pz orbital will undergo head to head overlapping. Fluorine molecules will not undergo sideways p-p orbital overlap.
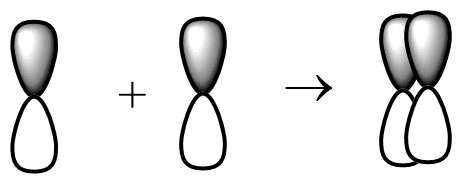
The rest of the overlapping is not possible in fluorine molecule as we can see that fluorine molecule as 9 atomic number so its electronic configuration will be like 1s22s22p5 . In fluorine, there is 2p partially filled orbital, so fluorine molecules come to each other to overlap and this overlapping of molecules will result in a covalent bond.
Hence, the correct option is (A).
Note:
The strength of a bond depends upon the extent of overlapping. As we can see that fluorine molecule has 2p5 partially-filled orbital, if we will consider this p orbital as normal 2p orbital instead of 2pz orbital then we will make mistake and will choose the wrong option.
