Question
Question: Fluorine is a non-metal. Will it give or take electrons?...
Fluorine is a non-metal. Will it give or take electrons?
Solution
Non-metals are elements which are present at the left side of the periodic table. There is some basic difference between them, non-metal is generally dull in appearance and less reactive than metals. Halogens come under the category of non-metals.
Complete answer:
In the periodic table, metal and nonmetal are placed at different places. However, specific differentiation between them is not done yet. Fluorine is the first member of the halogen family and known as non-metal. Atomic number of fluorine is 9, so when we write the electronic configuration of it according to Aufbau principle and Pauli exclusion principle, it will be represented as:
Electronic configuration of fluorine: 1s22s22p5
From the electronic configuration we see that, fluorine atom contains 7 electrons in its outermost shell. The outermost shell of fluorine is 2s and 2p. According to octet rule, every atom undergoes the chemical reaction to complete its outermost shell with 8 electrons to attain noble gas configuration. So as from the electronic configuration it is clear that a fluorine atom requires only one more electron to complete its octet to attain configuration of neon noble gas which is 1s22s22p6.
Electron gain enthalpy of fluorine is also very high so it is capable of attracting one electron from another atom to complete its octet.
Fluorine accepts one electron either by sharing to form a covalent bond or by attracting the electron from a less electronegative element to form fluoride ion.
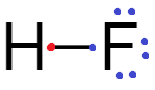
Therefore, fluorine atoms have a tendency to accept electrons.
Note:
Fluorine is highly reactive in nature and forms the strongest hydrogen bonding with hydrogen atoms. Fluorine has a tendency to react with non-metal like hydrogen as well as with metals like iron to form halides of halogen.
