Question
Question: Finding pH of the following samples by using pH paper/universal indicator: a.) Dilute Hydrochloric...
Finding pH of the following samples by using pH paper/universal indicator:
a.) Dilute Hydrochloric Acid
b.) Dilute NaOH solution
c.) Dilute Ethanoic Acid solution
d.) Lemon juice
e.) Water
Solution
The nature of the chemicals used in laboratories is either basic, acidic or neutral. This characteristic depends on the ions they release. A chemical is said to be acidic if it releases H+ ions and basic if it releases OH– ions in its aqueous solutions.
Complete step by step answer:
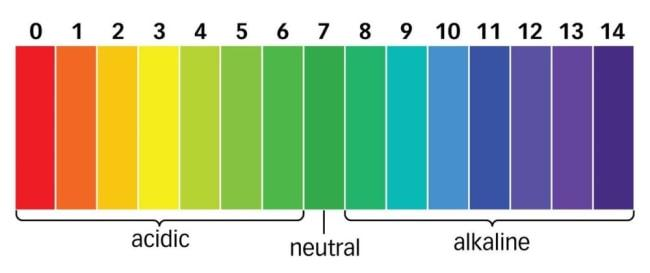
- The pH is the measure of the acidic or basic power of a solution. It is a scale for measuring hydrogen ion concentration in a solution.
- The pH scale varies from 0 to 14. Neutral solution has a pH of 7.
- pH is defined as the negative logarithm of H+ ions. It can be mathematically represented as
pH=−log10[H+]
pH scale
- To find the pH of the given chemicals, the apparatus required is as follows:
Test tubes
Test tube stand
Dropper
Glass rod
pH paper strips
pH paper colour chart
- First of all, arrange all the chemicals in clean, dry test tubes on the test tube stand.
- Secondly, put one or two drops of each test solution on pH paper strips using a glass rod.
- We will compare the pH using the pH scale chart from the observations given below:
| Sr. No. | Sample Solution | Colour appeared on pH paper | pH of the solution | Inference |
|---|---|---|---|---|
| 1. | Dilute HCl | Red | 1 | Strong acid |
| 2. | Dilute NaOH solution | Purple | 14 | Strong alkali |
| 3. | Dilute Ethanoic Acid solution | Yellow | 3 | Weak acid |
| 4. | Lemon Juice | Orange | 2 | Acid |
| 5. | Water | Green | 7 | Weak alkali |
Note: As pH depends upon H+ concentration and in an aqueous solution H+ and OH− ion concentrations are correlated, Therefore, every acidic and basic solution shows different colour at different pH .
