Question
Question: Find ‘X’ in the given reaction. \({C_2}{H_5} - O - {C_2}{H_5} + CO\xrightarrow[{{{150}^o}C}]{{B{F_...
Find ‘X’ in the given reaction.
C2H5−O−C2H5+COBF3150oCX
A. CH3COOH
B. CH3COOC2H5
C. CH3CH2COOC2H5
D. C3H7COOC2H5
Solution
Insertion reaction: In organic chemistry, when a chemical compound, molecule interposes itself into an existing bond of another molecule, then the reaction is known as insertion reaction. The general equation for insertion reaction is represented as follows:
A+B−C→B−A−C
Complete answer: For the given reaction conditions, BF3 catalyses the reaction and on heating the given ether in the presence of carbon monoxide, the insertion reaction takes place and the atoms of carbon monoxide are inserted between the C−O bond in ether and we get a yield of ester as a product. The reaction mechanism is given as follows:
Step-1: Lone pair of electrons on oxygen atom present in ether attack BF3 molecule and an intermediate product is formed. The reaction is shown as follows:
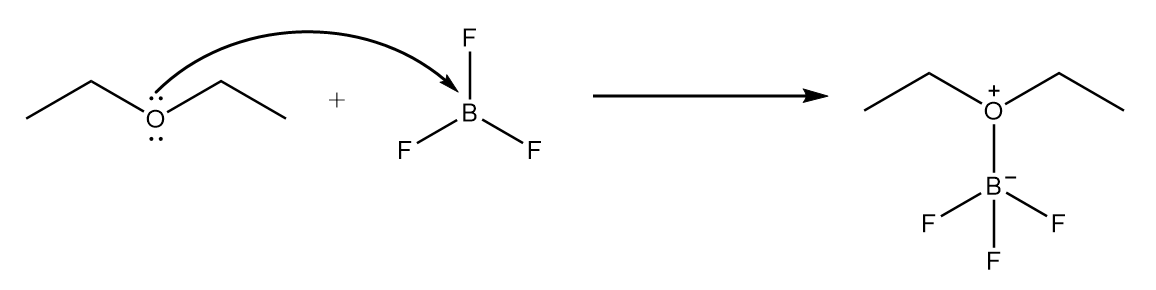
Step-2: Carbon atom of carbon monoxide attacks the carbon atom placed at adjacent position to oxygen atom and cleavage of C−O bond takes place. The reaction proceeds as follows:
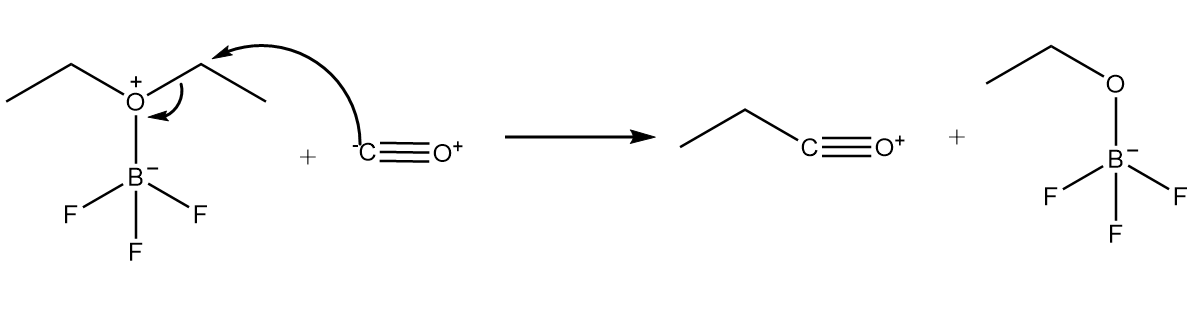
Step-3: As the positive charge on oxygen atoms makes the compound very unstable, so the cleavage of triple bond will take place and respective carbocation will be formed. The reaction proceeds as follows:
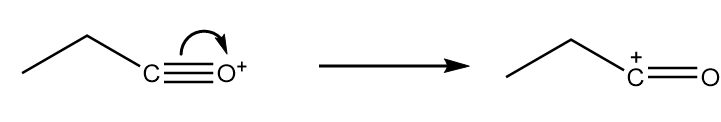
Step-4: The attack of −OC2H5 group from the anion formed in step-2 takes place on carbocation along with the removal of BF3 molecule. The reaction proceeds as follows:
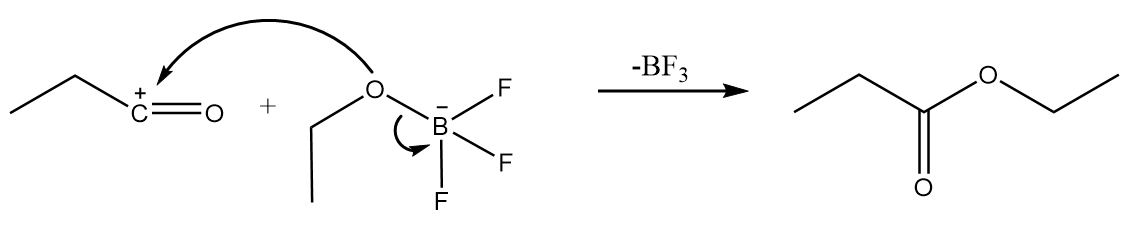
Hence, in the given reaction sequence, the structure of the product formed i.e., X is CH3CH2COOC2H5.
So, option (C) is the correct answer.
Note:
It is important to note that BF3 in the reaction acts as a neutral electrophile because it has six electrons in its valence shell and is considered as the electron deficient species due to its incomplete octet. Also, as the BF3 molecule is produced as the by-product in the final step of the reaction therefore it is considered as a catalyst for the reaction.
