Question
Question: Find x if the ratio of cis isomer to trans isomer is 1: x in \[[M(AA){{b}_{2}}{{c}_{2}}]\]....
Find x if the ratio of cis isomer to trans isomer is 1: x in [M(AA)b2c2].
Solution
When ligands are adjacent to one another in a metal complex, then it is a cis isomer. If the ligands are directly across from one another in the coordination sphere of the metal complex, then it is the trans isomer.
Complete answer:
Geometric isomerism in metal complexes is mainly shown by square planar and octahedral complexes.
[M(AA)b2c2] is an octahedral complex and can form geometrical isomers. An example for [M(AA)b2c2]type octahedral complex is [Co(en)(NH3)2Cl2]. Here en or ethylene diamine is a bidentate ligand. That is AA in the [M(AA)b2c2]refers to a bidentate ligand.
Now the isomers of [Co(en)(NH3)2Cl2] are
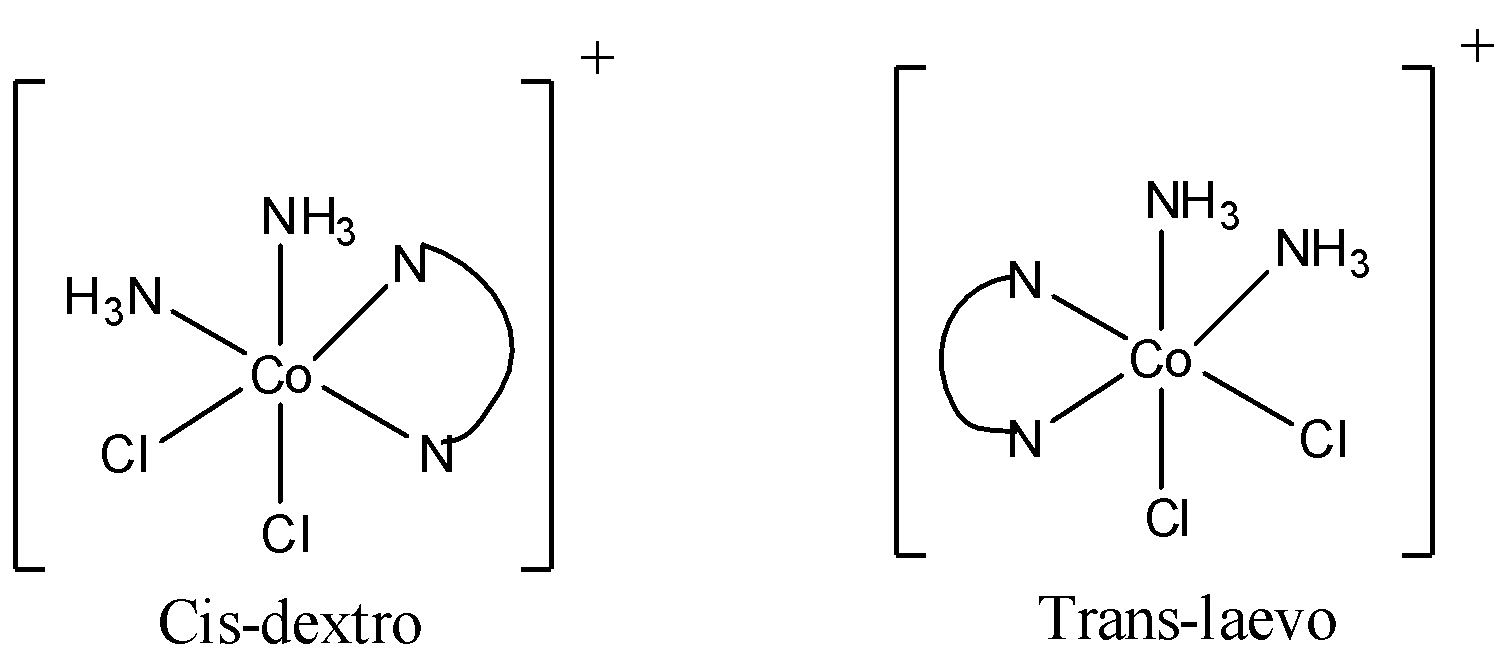
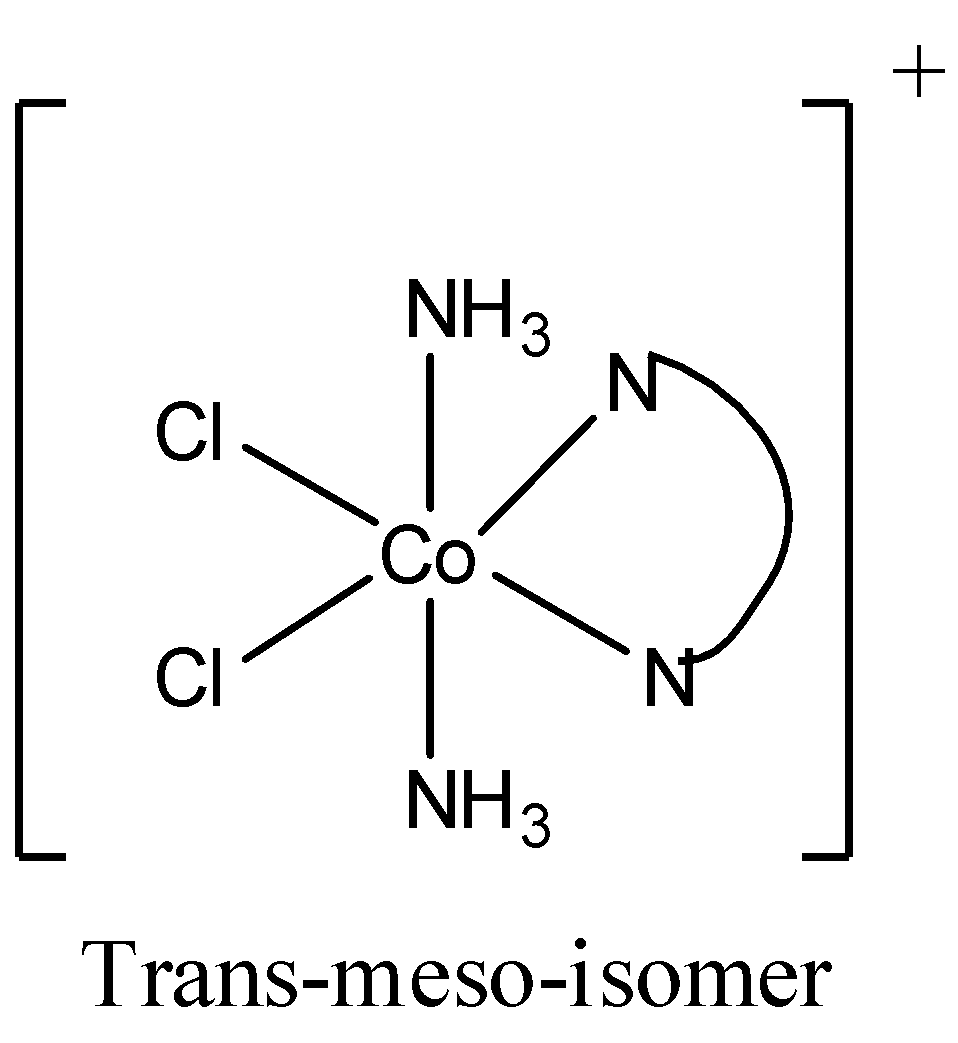
So there are 2 trans isomers, one is trans laevo and other is trans meso (it is optically inactive). Therefore, cis to trans isomer raion is 1:2. So, the x in 1:x is 2.
X=2
Additional Information: Stereoisomers are those which have the same ligands coordinated to the same metal ion and they have different arrangement of ligands in space. These are also called space isomers. Mostly 4-coordinated and 6-coordinated complexes show stereoisomerism. 2-coordinated and 3- coordinated complexes are rare and does not show any stereochemistry because all the ligands are equidistant. 4-coordinated complexes have either square planar or tetrahedral structures. Only square planar complexes show geometrical isomerism. Some types of octahedral complexes show geometrical isomerism.
Note: Stereoisomers are designated as D or dextro and L or leavo according to the direction in which the substance can rotate polarised light. If the polarised light is rotated to the right, then it is D isomer and if the polarised light is rotated to the left, then it is L-isomer. These are optically active. There is meso isomer which is inactive because it can form racemic mixtures.
