Question
Question: Find value of Q from following equations. (i) $C_{(s)}+O_{2(g)} \longrightarrow CO_{2(g)} \Delta H ...
Find value of Q from following equations.
(i) C(s)+O2(g)⟶CO2(g)ΔH=QkJ (ii) C(s)+21O2(g)⟶CO2(g)ΔH=2xkJ (iii) C(s)+21O2(g)⟶CO2(g)ΔH=2ykJ
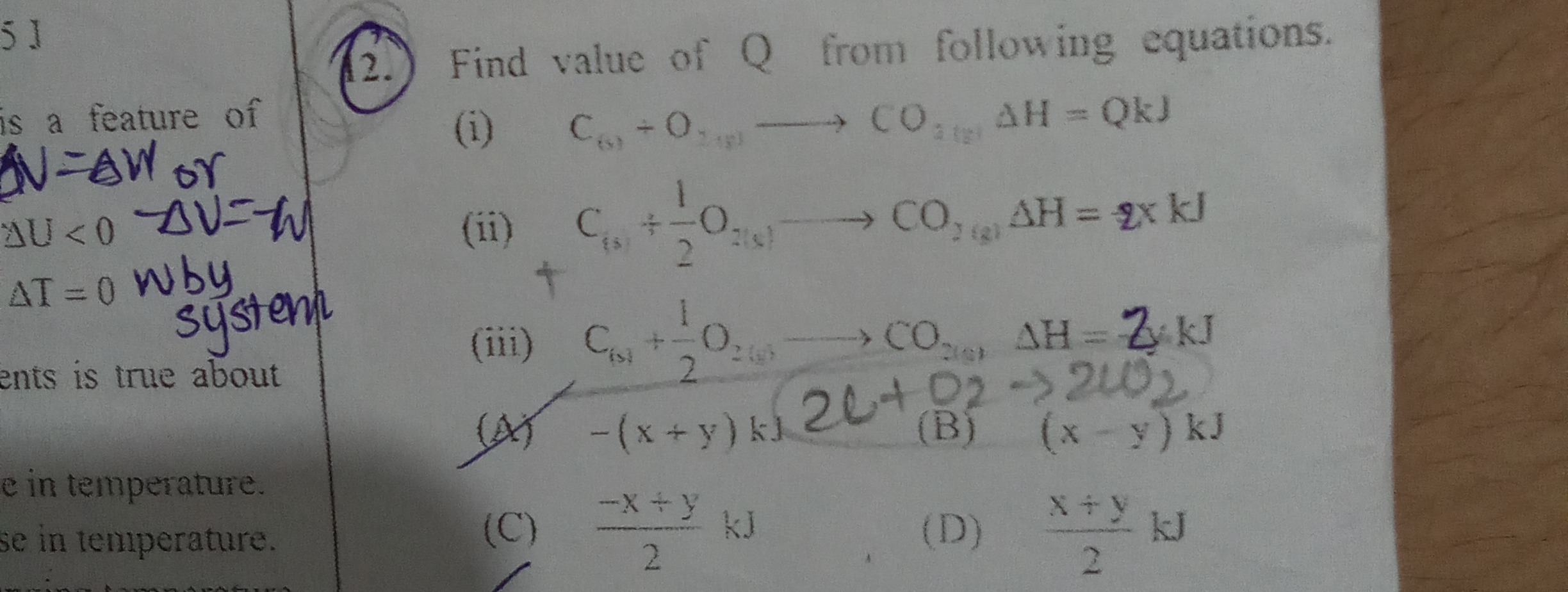
A
-(x+y) kJ
B
(x-y) kJ
C
2−x+ykJ
D
2x+ykJ
Answer
-(x + y) kJ
Explanation
Solution
Solution:
For the formation reaction
C(s)+21O2(g)⟶CO2(g)
the two experiments yield the energy changes 2x kJ and 2y kJ. Interpreting these as corresponding to –x kJ and –y kJ per mole (after accounting for the sign convention), we take the average for the half–reaction:
Δ1/2=−(x+y)/2
Now, the full reaction,
C(s)+O2(g)⟶CO2(g)
is exactly twice the half–reaction so that
Q=2×[−(x+y)/2]=−(x+y)kJ
