Question
Question: Find total number of reaction with correct major product: ```latex \begin{array}{ccccccccc} & CH_3 ...
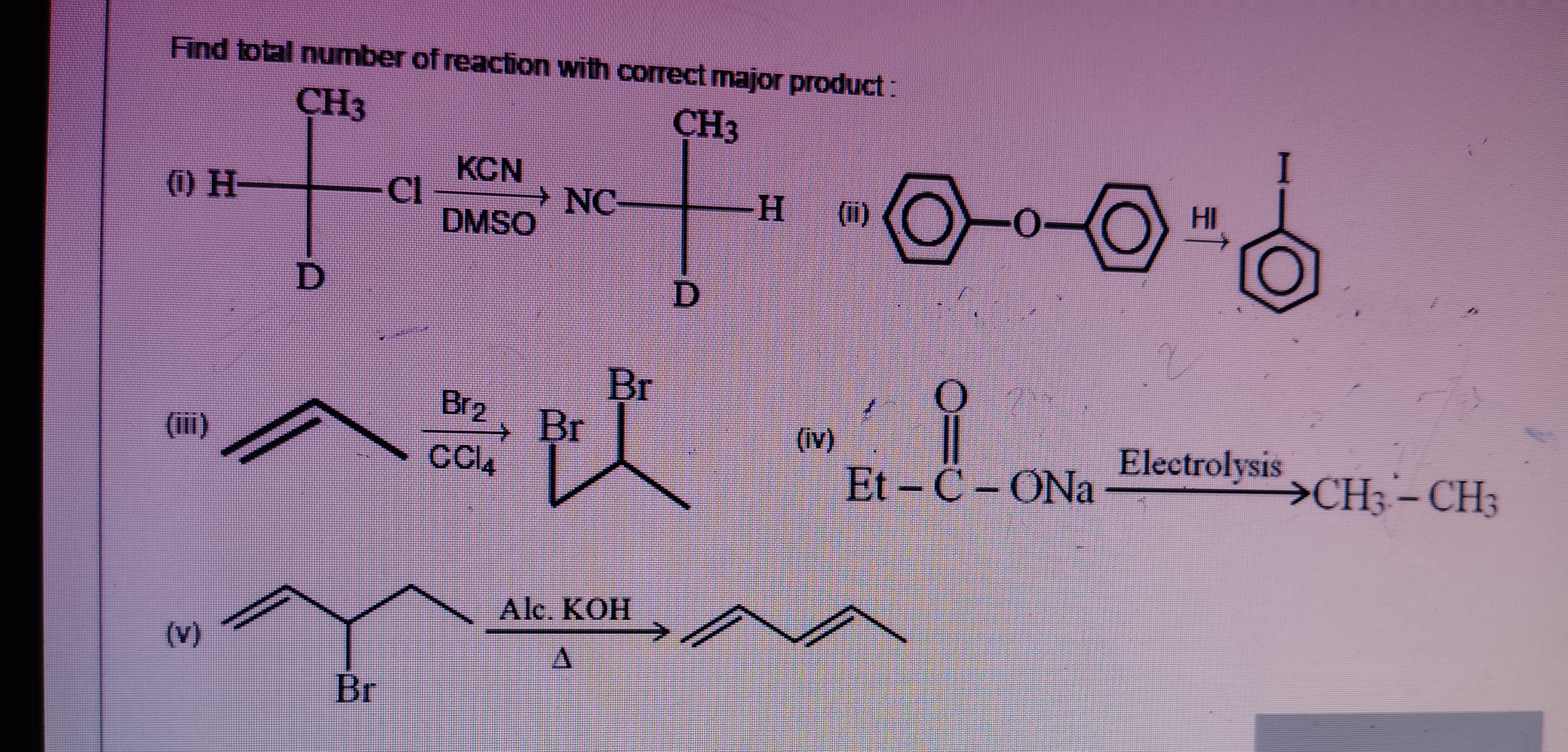
Find total number of reaction with correct major product:
\begin{array}{ccccccccc} & CH_3 & & & & & & CH_3 & \\ (i) & H- \underset{|}{C} & \xrightarrow[\text{DMSO}]{\text{KCN}} & NC- \underset{|}{C} -H & (ii) & \bigcirc -O- \bigcirc & \xrightarrow{HI} & \bigcirc \xrightarrow{HI} \bigcirc -I \\ & D & Cl & & D & & & \\ \end{array}
\begin{array}{ccccc} & & Br & & \\ (iii) & \underset{|}{} \xrightarrow[\text{CCl}_4]{\text{Br}_2} & Br- \underset{|}{C} -Br & (iv) & Et - \underset{||}{C} - ONa \xrightarrow[\text{}]{Electrolysis} CH_3-CH_3 \\ \end{array}
\begin{array}{ccccc} (v) & \underset{|}{} - Br \xrightarrow[\Delta]{\text{Alc. KOH}} \underset{|}{} \\ \end{array}
1
Solution
Here is the analysis of each reaction:
(i) The reactant is (R)-1-chloro-1-deuterioethane. The reaction is with KCN in DMSO. This is an SN2 reaction. SN2 reactions at a chiral center proceed with inversion of configuration (Walden inversion). The reactant has R configuration. The product of SN2 reaction should have S configuration. The given product has R configuration. Therefore, the product shown is incorrect.
(ii) The reactant is diphenyl ether. The reaction is with HI. Cleavage of ethers with HI leads to the formation of alkyl halides and alcohols. In the case of aryl ethers, the C-O bond connected to the phenyl ring is strong due to resonance and is not easily cleaved. The other C-O bond is cleaved. For symmetrical diphenyl ether, cleavage with HI should produce phenol and iodobenzene. Both are formed. The diagram shows iodobenzene as the product. While iodobenzene is a product, it is not the only product, and it's not necessarily the major product in all contexts. However, if the question is asking if the shown product is a correct product formed in the reaction, then iodobenzene is indeed formed. But the question asks for the correct major product. In the cleavage of symmetrical diphenyl ether with HI, both phenol and iodobenzene are formed in a 1:1 ratio. Therefore, showing only iodobenzene as the major product is incorrect.
(iii) The reactant is but-1-ene (CH2=CH−CH2−CH3). The reaction is with Br2 in CCl4. This is an electrophilic addition of bromine to an alkene, which results in the formation of a vicinal dibromide. The product should be 1,2-dibromobutane (CH2Br−CHBr−CH2−CH3). The given product is 1,2-dibromo-2-methylpropane (CH2Br−C(Br)(CH3)2), which is obtained from the addition of Br2 to 2-methylpropene (CH2=C(CH3)2). Therefore, the product shown is incorrect.
(iv) The reactant is sodium propanoate (CH3CH2COO−Na+). The reaction is electrolysis. This is Kolbe's electrolytic synthesis, which results in the formation of an alkane by dimerization of alkyl radicals formed from the decarboxylation of carboxylate ions. From sodium propanoate, the ethyl radical (CH3CH2⋅) is formed, which then dimerizes to form butane (CH3CH2−CH2CH3). The given product is ethane (CH3−CH3). Ethane is obtained from the electrolysis of sodium acetate (CH3COO−Na+). Therefore, the product shown is incorrect.
(v) The reactant is 2-bromopentane (CH3−CH2−CH(Br)−CH2−CH3). The reaction is with alcoholic KOH and heat. This is an E2 elimination reaction, which leads to the formation of an alkene. Elimination of HBr can occur from the second carbon (with Br) and the first carbon (with H) to form pent-1-ene (CH2=CH−CH2−CH2−CH3), or from the second carbon (with Br) and the third carbon (with H) to form pent-2-ene (CH3−CH=CH−CH2−CH3). According to Zaitsev's rule, the more substituted alkene is the major product. Pent-2-ene is disubstituted, while pent-1-ene is monosubstituted. Thus, pent-2-ene is the major product. The given product is pent-2-ene (CH3−CH=CH−CH2−CH3). Therefore, the product shown is correct.
Based on the analysis, only reaction (v) shows the correct major product.
Reaction (i) is an SN2 reaction which should cause inversion of configuration, but the product shows retention. Incorrect. Reaction (ii) is cleavage of diphenyl ether with HI. Products are phenol and iodobenzene. Showing only iodobenzene as major product is incorrect. Reaction (iii) is addition of Br2 to but-1-ene. Product should be 1,2-dibromobutane, but 1,2-dibromo-2-methylpropane is shown. Incorrect. Reaction (iv) is Kolbe's electrolysis of sodium propanoate. Product should be butane, but ethane is shown. Incorrect. Reaction (v) is E2 elimination of 2-bromopentane with alcoholic KOH. Major product is pent-2-ene (Zaitsev's rule), which is shown. Correct. Total number of reactions with correct major product is 1.
