Question
Question: Find total number of compounds which can produced by Wurtz reaction in good yeild...
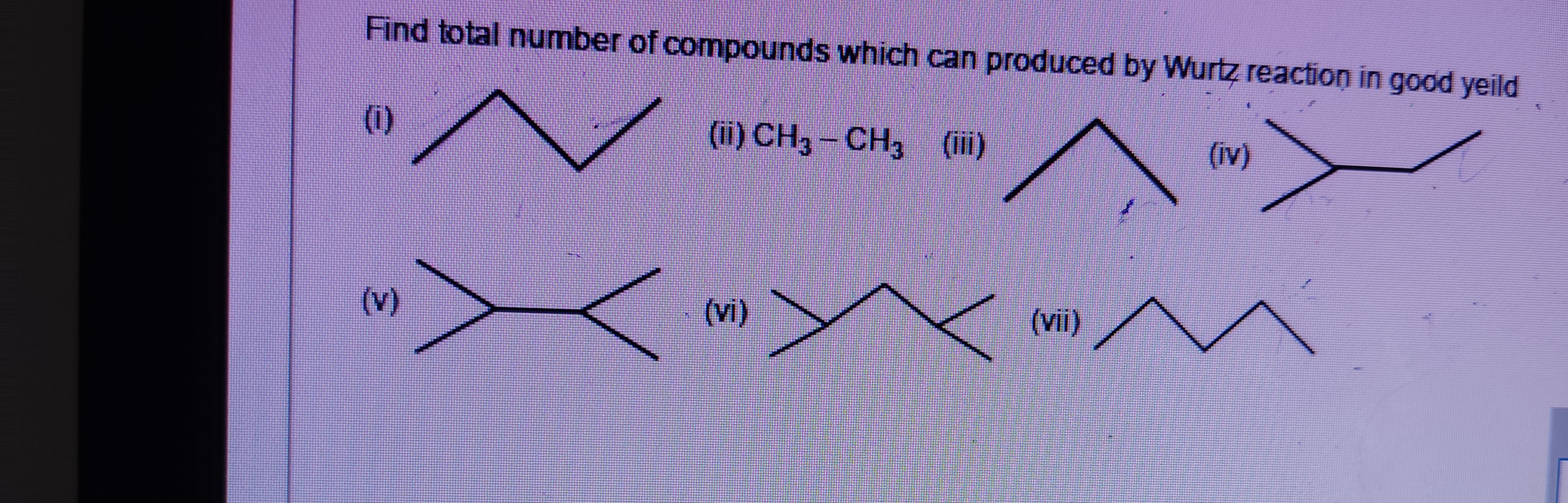
Find total number of compounds which can produced by Wurtz reaction in good yeild
3
Solution
Wurtz reaction is primarily used for the synthesis of symmetrical alkanes by coupling of two identical alkyl halides in the presence of sodium metal in dry ether. The reaction is given by 2RX+2Na→R−R+2NaX. Wurtz reaction works best with primary alkyl halides, giving good yields of symmetrical alkanes. Secondary and tertiary alkyl halides give lower yields due to competing elimination reactions. When a mixture of different alkyl halides is used, a mixture of three possible alkanes is formed, leading to poor yield of any single product. Therefore, to obtain a compound in good yield by Wurtz reaction, it should be a symmetrical alkane formed by coupling of two identical primary alkyl groups.
Let's analyze the given compounds:
(i) Butane (CH3−CH2−CH2−CH3). This is a symmetrical alkane formed by coupling of two ethyl groups (CH3−CH2−). Ethyl halide is a primary alkyl halide. So, butane can be produced in good yield from ethyl halide by Wurtz reaction: 2CH3CH2X+2Na→CH3CH2CH2CH3+2NaX.
(ii) Ethane (CH3−CH3). This is a symmetrical alkane formed by coupling of two methyl groups (CH3−). Methyl halide is a primary alkyl halide. So, ethane can be produced in good yield from methyl halide by Wurtz reaction: 2CH3X+2Na→CH3CH3+2NaX.
(iii) Propane (CH3−CH2−CH3). This is not a symmetrical alkane formed by coupling of identical alkyl groups. To form propane, we would need to couple a methyl group and an ethyl group, which would require a mixture of methyl halide and ethyl halide, leading to a mixture of ethane, propane, and butane, and thus poor yield of propane.
(iv) 2-methylpropane (CH3−CH(CH3)−CH3). This is not a symmetrical alkane formed by coupling of identical alkyl groups.
(v) 2,3-dimethylbutane ((CH3)2CH−CH(CH3)2). This is a symmetrical alkane formed by coupling of two isopropyl groups ((CH3)2CH−). Isopropyl halide is a secondary alkyl halide. Wurtz reaction with secondary alkyl halides gives lower yields due to competing elimination reactions. Thus, 2,3-dimethylbutane is not produced in good yield by Wurtz reaction.
(vi) 3-methylpentane (CH3−CH2−CH(CH3)−CH2−CH3). This is not a symmetrical alkane formed by coupling of identical alkyl groups.
(vii) Hexane (CH3−CH2−CH2−CH2−CH2−CH3). This is a symmetrical alkane formed by coupling of two propyl groups (CH3−CH2−CH2−). Propyl halide is a primary alkyl halide. So, hexane can be produced in good yield from propyl halide by Wurtz reaction: 2CH3CH2CH2X+2Na→CH3CH2CH2CH2CH2CH3+2NaX.
Therefore, the compounds which can be produced by Wurtz reaction in good yield are (i) Butane, (ii) Ethane, and (vii) Hexane. Total number of such compounds is 3.
