Question
Question: Find the total no.of aromatic and non-aromatic compounds ...
Find the total no.of aromatic and non-aromatic compounds
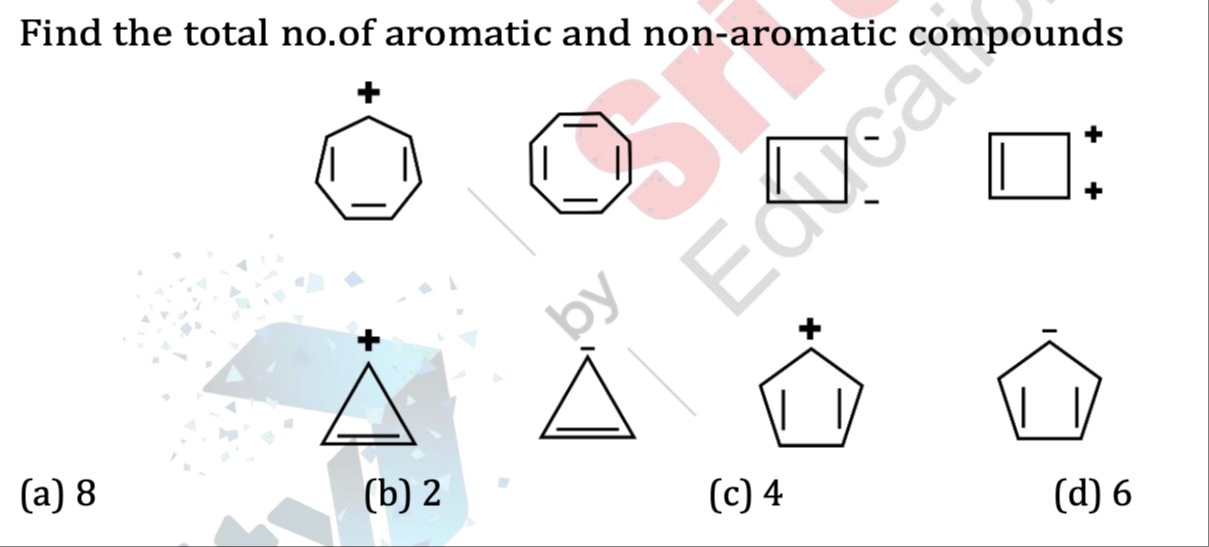
8
2
4
6
There are 5 aromatic compounds and 3 non–aromatic compounds.
(Since 5 + 3 = 8 total, option (a) 8 is the total number of structures.)
Solution
We must decide for each planar cyclic structure if its π‐system obeys Hückel’s rule. (Recall that for a fully conjugated, planar ring the number of π–electrons must equal 4n+2 for aromaticity; if it equals 4n the compound is antiaromatic and is usually considered “non‐aromatic”. In a cyclic system made only of sp² carbons, a positive charge means a missing electron on that atom while a negative charge means an extra electron.)
A good way to “count” is to assume that each carbon in a neutral ring supplies one electron; then adjust for the charges.
Let the compounds be numbered as in the figure (reading left‐to‐right, top then bottom):
-
A 7‐membered ring with 3 double bonds and a + charge:
Count = 7 – 1 = 6 → 6 = 4×1 + 2 → Aromatic.
-
An 8‐membered ring with 4 double bonds (no charge):
Count = 8 → 8 = 4×2 → (Antiaromatic, hence considered non‐aromatic.)
-
A 4‐membered ring with one double bond and two – charges:
Count = 4 + 2 = 6 → Aromatic.
-
A 4‐membered ring with one double bond and two + charges:
Count = 4 – 2 = 2 → 2 = 4×0+2 → Aromatic.
-
A 3‐membered ring with one double bond and a + charge (the famous cyclopropenium ion):
Count = 3 – 1 = 2 → Aromatic.
-
A 3‐membered ring with one double bond and a – charge:
Count = 3 + 1 = 4 → 4 = 4×1 → Antiaromatic (thus non‐aromatic).
-
A 5‐membered ring with two double bonds and a + charge (the cyclopentadienyl cation):
Count = 5 – 1 = 4 → 4 electrons → (Antiaromatic.)
-
A 5‐membered ring with two double bonds and a – charge (the cyclopentadienyl anion):
Count = 5 + 1 = 6 → Aromatic.
Aromatics (π–electron count 4n+2): Compounds 1, 3, 4, 5 and 8 (5 compounds).
Non‐aromatics (here antiaromatic, having 4n electrons): Compounds 2, 6 and 7 (3 compounds).
Thus, out of the eight structures, 5 are aromatic and 3 are non‐aromatic. (Sometimes antiaromatic compounds are called “non–aromatic” in exam questions.)
Minimal Explanation
• Count π–electrons using “each carbon =1” adjusted by charges.
• 7–membered (+1): 7–1 =6 (aromatic), 8–membered: 8 (antiaromatic),
• 4–membered with (– –): 4+2=6 (aromatic) and with (++): 4–2=2 (aromatic),
• 3–membered: cation: 3–1=2 (aromatic), anion: 3+1=4 (non–aromatic),
• 5–membered: cation: 5–1=4 (non–aromatic), anion: 5+1=6 (aromatic).
→ Aromatic: 1, 3, 4, 5, 8 (5 total); Non–aromatic: 2, 6, 7 (3 total).
