Question
Question: Find the total no. of sigma and pi bonds in the given compound. 
Solution
Nitrogen molecule has a valency of 3. In this compound, the −NH2 group is attached to the benzene group. It has one lone pair to form pi bonds as well with other atoms. Aniline has nitrogen atoms and carbon atoms with sp3 and sp2 hybridization.
Complete step by step answer:
The Lewis structure for aniline consists of a nitrogen atom that has signal bonds with two hydrogens and one carbon of benzene ring. Lewis structure is based on the octet rule which states that there should be eight electrons in the outermost shell or orbit of an atom for the molecule to be stable.
In the benzene ring, the carbon atoms are sp2 hybridization. Each carbon is connected with another two carbon atoms and one hydrogen atom. In this case, each carbon has one pi bond with one carbon. So, there are a total of 3 pi bonds, 6 carbon atoms are attached and form the hexagonal ring. So, there are a total of 6 sigma bonds. Each hydrogen atom has one sigma bond, there are a total of 7 hydrogen atoms so 7 sigma bonds are formed by 7 hydrogens.
So, the number of sigma bonds is 14 and the number of pi bonds is 3.
Additional information:
Aniline is an aromatic amine. The basicity of the aromatic amine is depending upon the availability of the lone pair. The higher the lone pair availability, the higher the donating ability of the lone pair as well as the accepted tendency of hydrogen ions.
In the case of aromatic amine, the lone pair undergoes conjugation with the benzene. As a result, lone pair availability decreases as well as the basicity. The resonance is shown below.
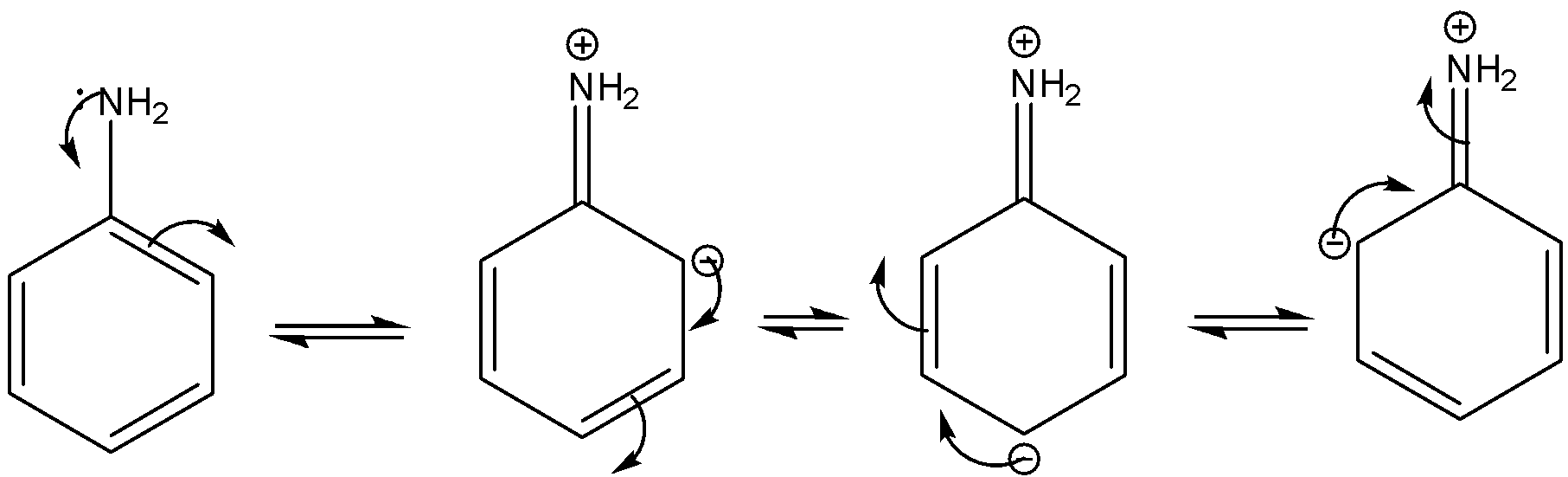
Note: According to the concept of Lewis acid-base theory the compound or molecule can accept hydrogen ion is known as base. Higher the tendency to accept hydrogen ion higher will be the basicity of that compound. In the case of the aliphatic amine with increasing the inductive effect of the alkyl group the electron density of the nitrogen increases as well as the basicity. In the case of aniline due to conjugation, the lone pair density is less than that of methylamine. Due to this reason, aniline is less basic than methylamine.
