Question
Question: Find the sum of nodal plane of \({{\pi }}_{{\text{d - d}}}^ * \)anti-bonding molecular orbital....
Find the sum of nodal plane of πd - d∗anti-bonding molecular orbital.
Solution
The d-orbitals having four lobes overlap sidewise to form a pi bond. When these d-orbitals having four lobes overlap in a destructive manner, the πd - d∗anti-bonding molecular orbital. The plane where the electron density is not found represents a node. By the picture of molecular orbitals, the nodal plane can be determined.
Complete Step by step answer: Two atomic orbital combines to form two molecular orbitals. Out of these two molecular orbital one is known as bonding which has low energy and the second is known as antibonding which has high energy. s-and one p-orbital forms sigma bond and remaining two p-orbitals form pi bonding.
The d-orbital is a set of five denigrate orbitals named as dz2, dX2−Y2, dXZ,dYZ, anddXY.
The structure of all five d-orbitals is represented as follows:
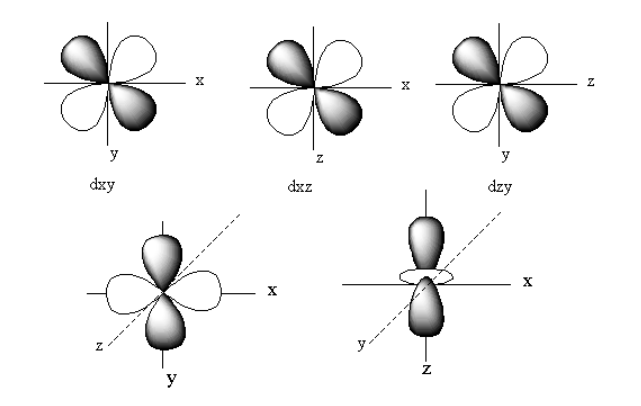
The pi bond is formed by side-on overlapping of the two d-orbitals. When two d-orbitals overlap out of phase the antibonding orbitals form. The antibonding molecular orbitals are denoted by superscript ‘∗’.
The structure of the bonding πd - dorbitals and πd - d∗ anti-bonding is as follows:
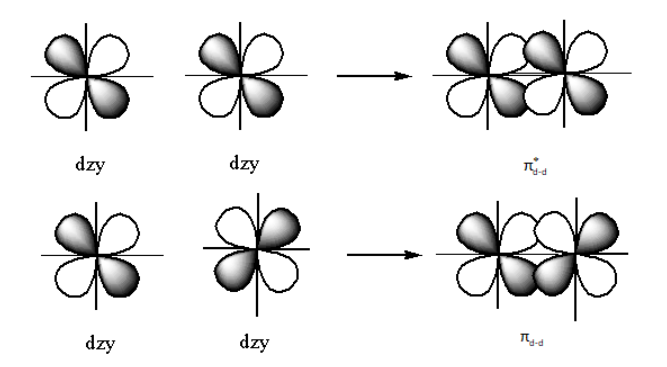
The nodal plane is the plane where the probability of finding the electrons is zero.
The nodal plane in πd - d∗ anti-bonding is as follows:
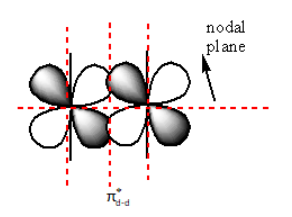
The red dashed lines in the above diagram show the nodal plane. So, the πd - d∗ anti-bonding molecular orbitals have a total of four nodal planes.
Therefore, the sum of the nodal plane of πd - d∗anti-bonding molecular orbital is four.
Note: At node the sign of orbital changes. Here, the dark colour and light colour of the orbitals shows the different phases of the orbitals. So, the change of colour (dark to light or light to dark) shows the phase change. Pi bonding orbitals form by constructive overlapping of the two d-orbitals. The nodal plane in pi bonding πd - d molecular orbital is three.
