Question
Question: Find the structure of B in the given organic reaction. 
Solution
Hint : Cannizzaro reaction: An organic reaction which involves disproportionation of two non-enolizable carbonyl compounds in the presence of a base and formation of a carboxylic acid along with a primary alcohol takes place. This reaction is a type of redox reaction which involves transfer of a hydride from one molecule to another.
Complete Step By Step Answer:
The two main conditions which are necessary for a carbonyl compound to undergo Cannizzaro reaction are as follows:
It must not have any alpha hydrogen.
The reaction must be carried out in basic conditions.
As the given compound does not have any alpha hydrogen and two aldehyde groups are present within the same molecule, an intramolecular Cannizzaro reaction will take place. The reaction mechanism will be as follows:
Step-1: Hydroxide ion from base will act as nucleophile and will attack at the electrophilic carbonyl centre of the given compound. The reaction will take place as follows:
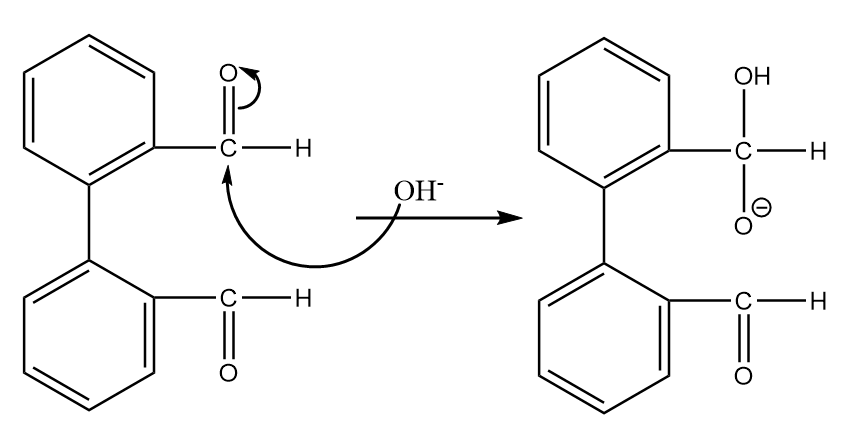
Step-2: The transfer of hydride ions will take place and an aldehyde group will be converted into a carboxylic group. The reaction proceeds as follows:
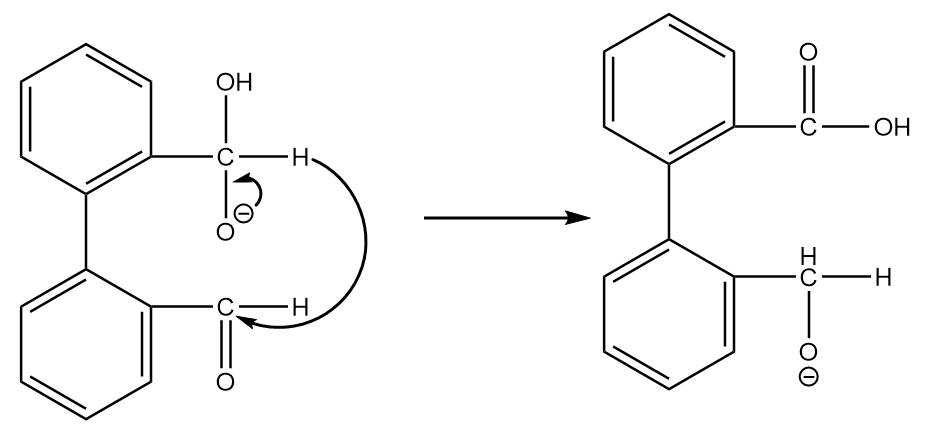
Step-3: On acidic workup of the reaction, the second aldehyde group converts into primary alcohol. The reaction proceeds as follows:
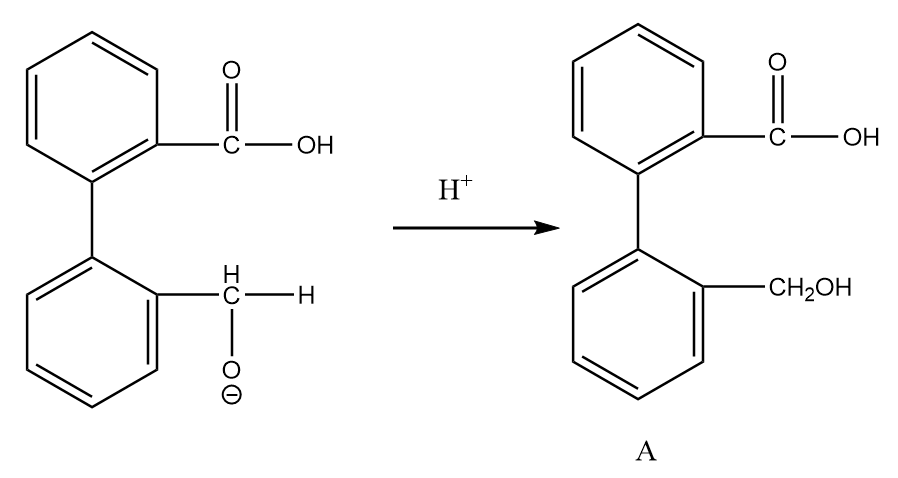
Now, on further heating in the acidic medium, the removal of the water molecule takes place and the final product is formed. The reaction proceeds as follows:
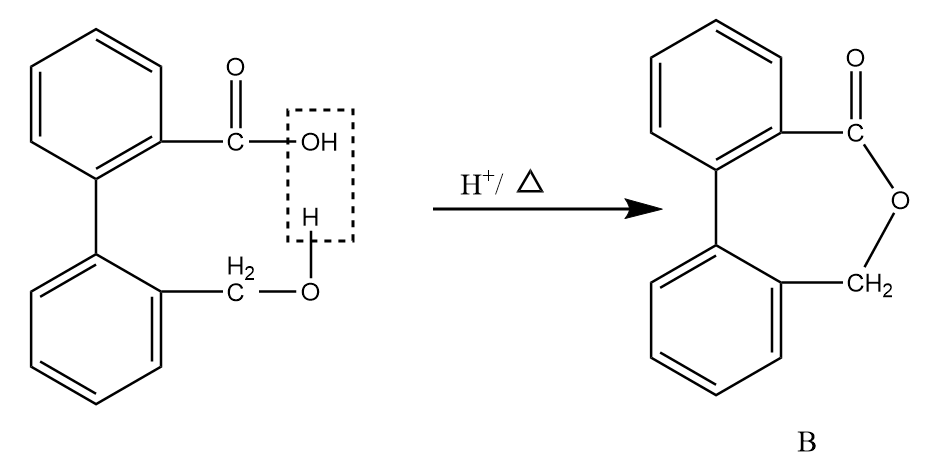
Hence, the compound B formed after given reaction sequence is:
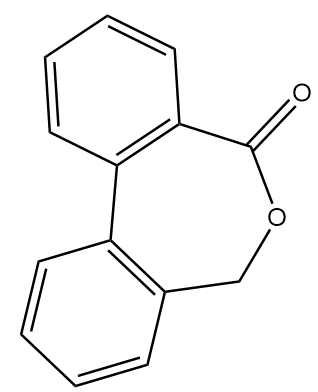
Note :
Remember that the compounds which consist of alpha hydrogens i.e., hydrogen atoms bonded to the carbon adjacent to the carbonyl group, cannot undergo Cannizzaro reaction. Instead, it shows aldol condensation under the same reaction conditions.
