Question
Question: Find the structural formula of benzene. * A. 
-
B.
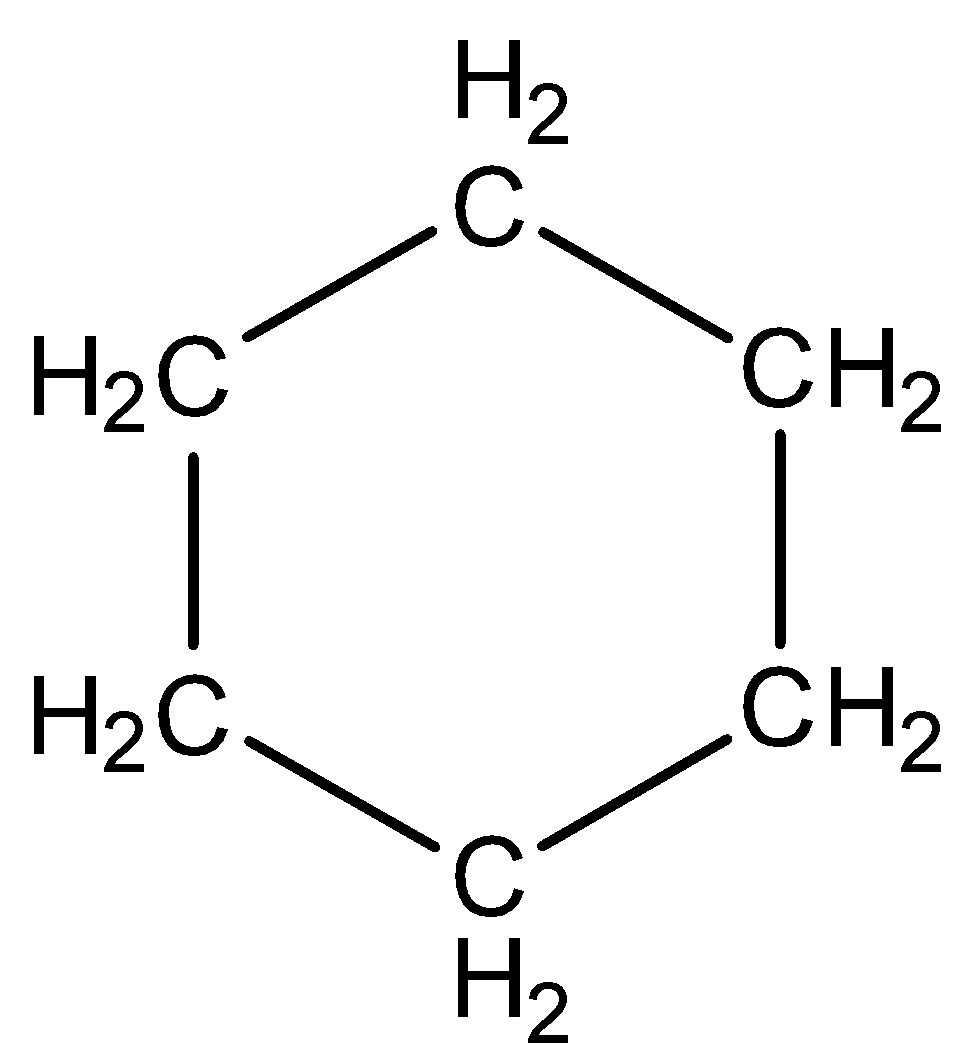
-
C.
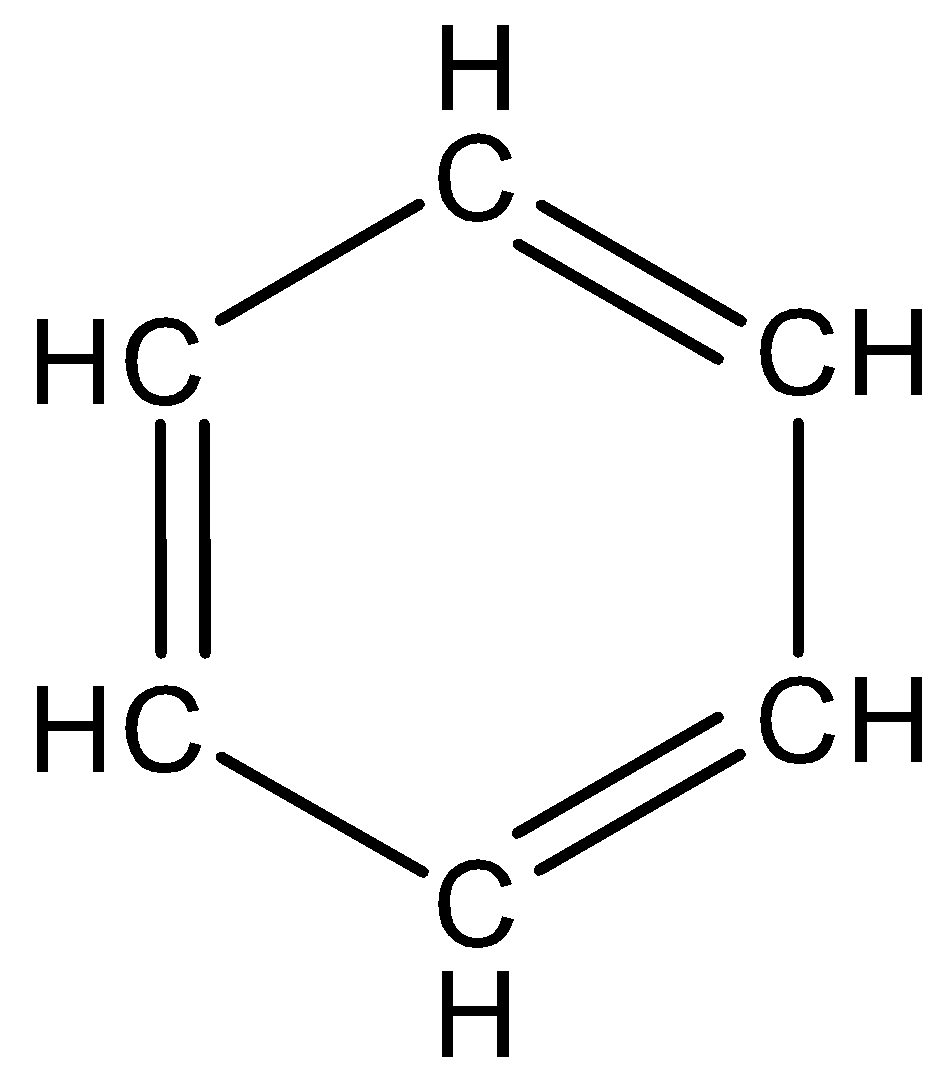
-
D.
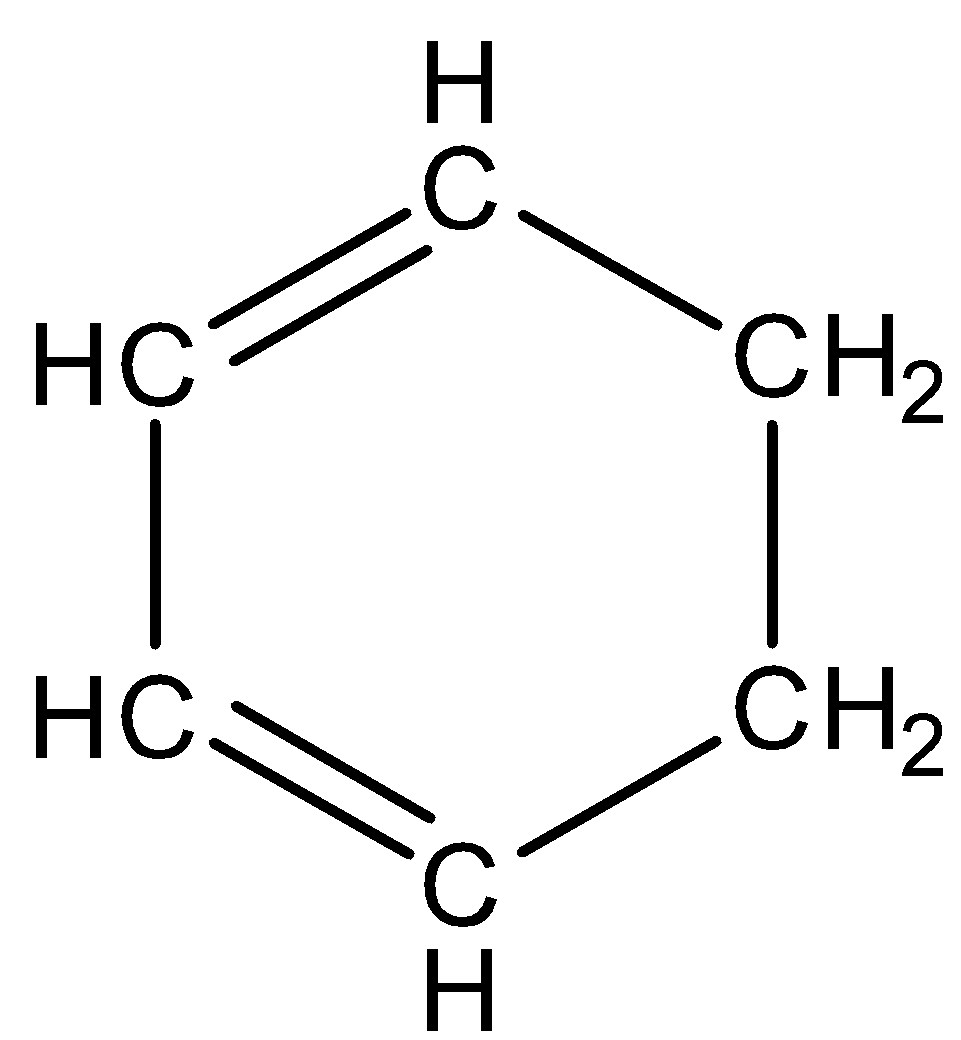
Solution
We know that the molecular formula of benzene is C6H6 and it is a cyclic compound and it has alternate double bonds.
Complete step by step answer:
First, we see about benzene
The molecular formula of benzene is C6H6. Benzene is the simplest aromatic hydrocarbon. It is a colorless, flammable liquid having a gasoline like odor. It is mainly used for producing polystyrene. Benzene is highly toxic and also a carcinogen and may cause leukemia.
We know the structure of benzene.
The structure of benzene is,
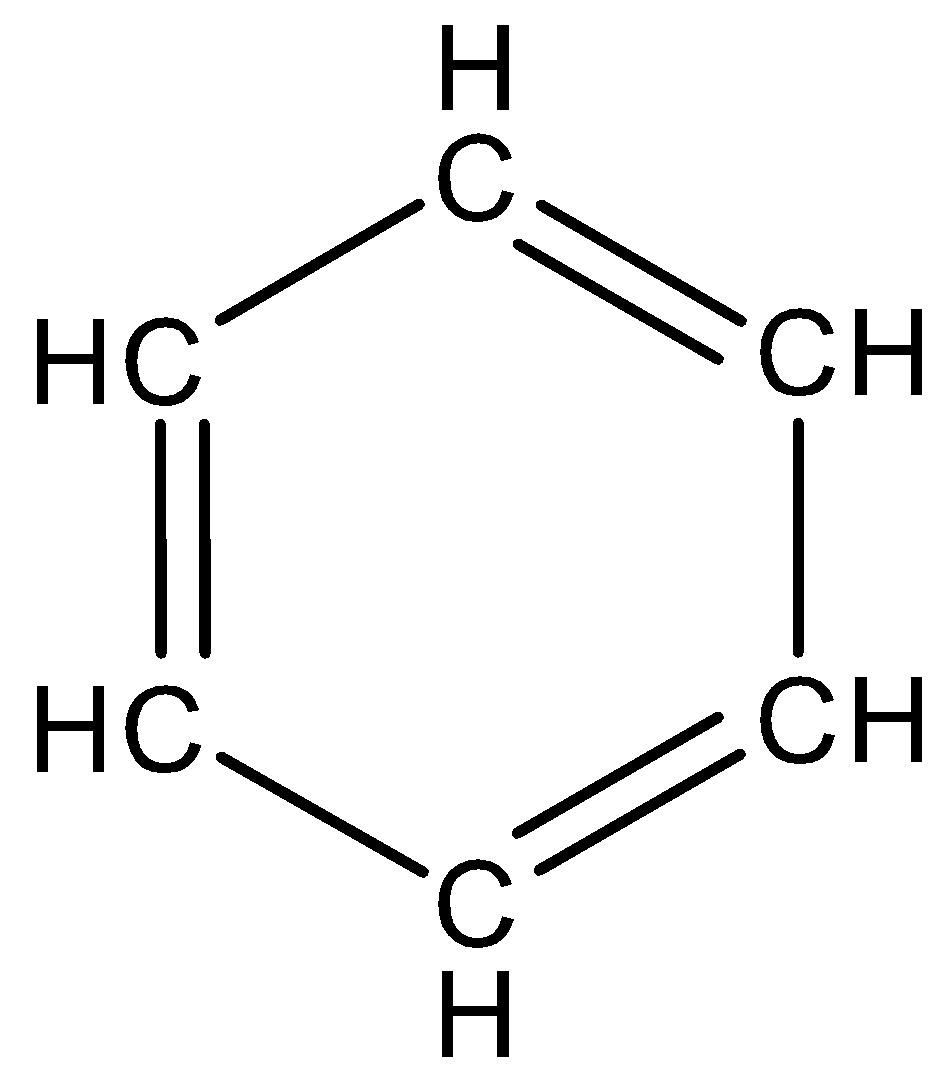
Benzene has six hydrogen and six carbon atoms and the molecular weight of benzene is 78.11g/mol. the above structure of benzene has six carbon atoms in a hexagon ring and has alternate double bonds. The double bonds are called conjugated double bonds and a circle is used alternatively inside the hexagon to represent six pi electrons.
Benzene is a planar molecule and three electrons are delocalized above and below the plane. The delocalization of electrons makes the benzene ring more stable and the bond length of all C−C bonds is 1.47A∘ and the bond angle is 120∘.
∴The correct answer is option (C).
Note:
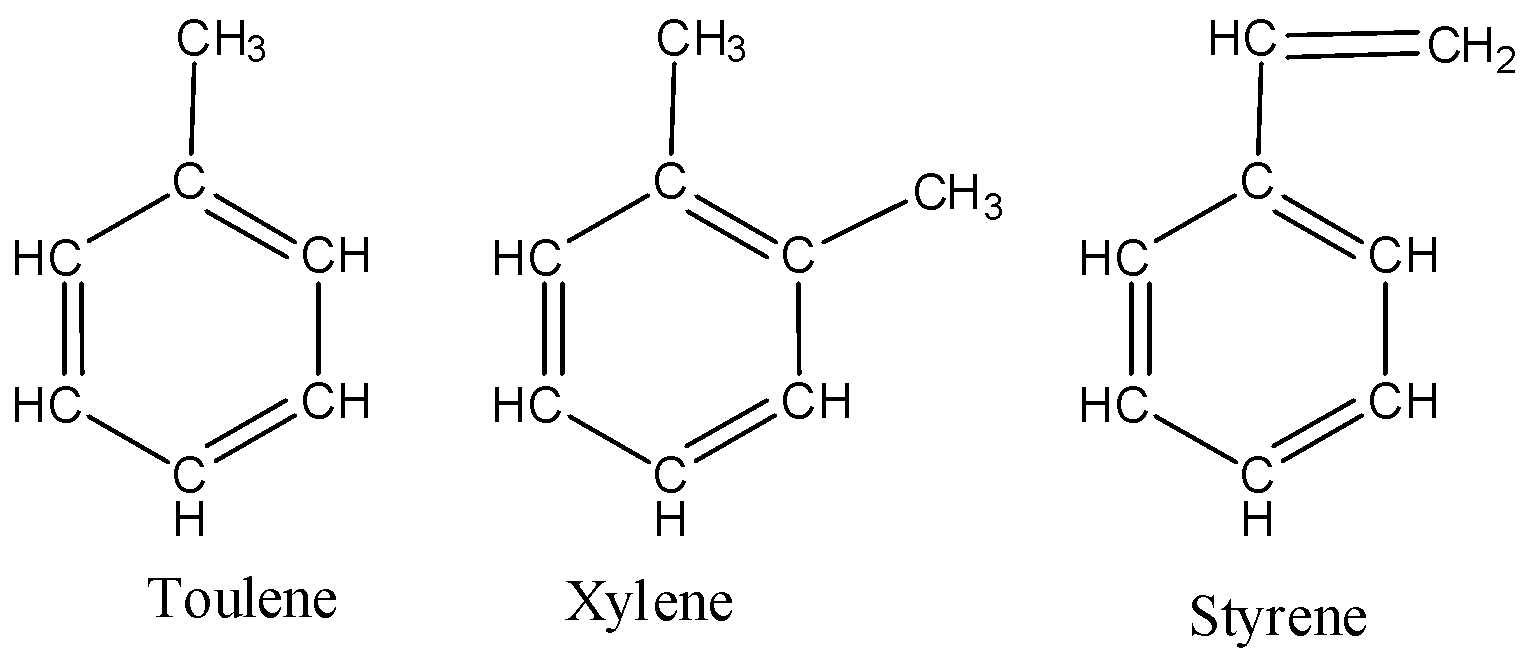
We must know that benzene readily undergoes substitution reactions. There are many derivatives of benzene which are by the replacement of one of the hydrogen atoms with another substituent. Some of the derivatives of benzene are toluene, xylene, and styrene. Benzene does not show an additional reaction.
The structure of toluene, xylene, and styrene are as follows,