Question
Question: Find the product of the following reaction: 
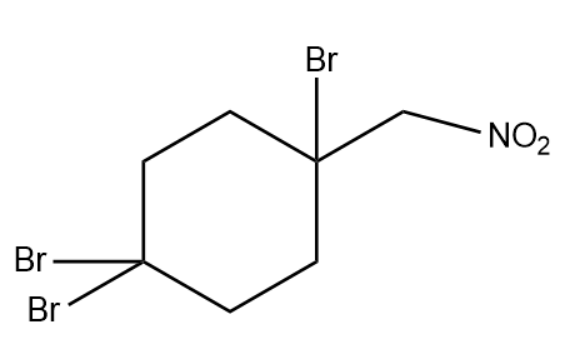
(1) 
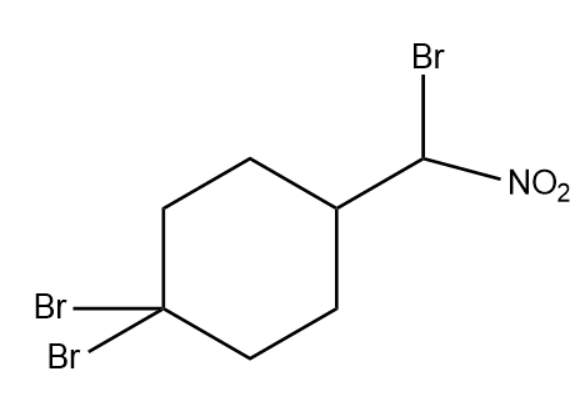
(2) 
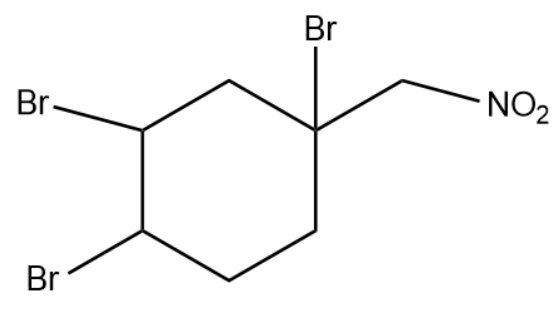
(3) 
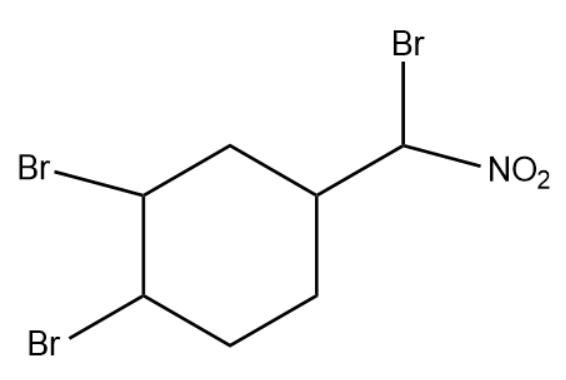
(4)
Solution
Hint : In this question we are doing an additional reaction. HBr when added to an alkene, it will undergo an additional reaction which is governed by Markovnikov's Rule.
We all know Markovnikov's Rule states that when a protic acid such as HBr is added to an asymmetric alkenes then the hydrogen of the acid will attach to that carbon having more number of hydrogens and the halide group will attach to the carbon having greater number of substituents.
Complete Step By Step Answer:
We have two double bonds in the reactant and we also have 2 moles of HBr which means that both the double bonds will become saturated (turns into a single bond).
Now to know where the Br will be substituted we have to find the most substituted carbon in the double bond.
The positions marked with the dots are the most substituted carbon in the double bond.
Thus Br will be substituted in those positions and hydrogen on the other carbon of the double bond.
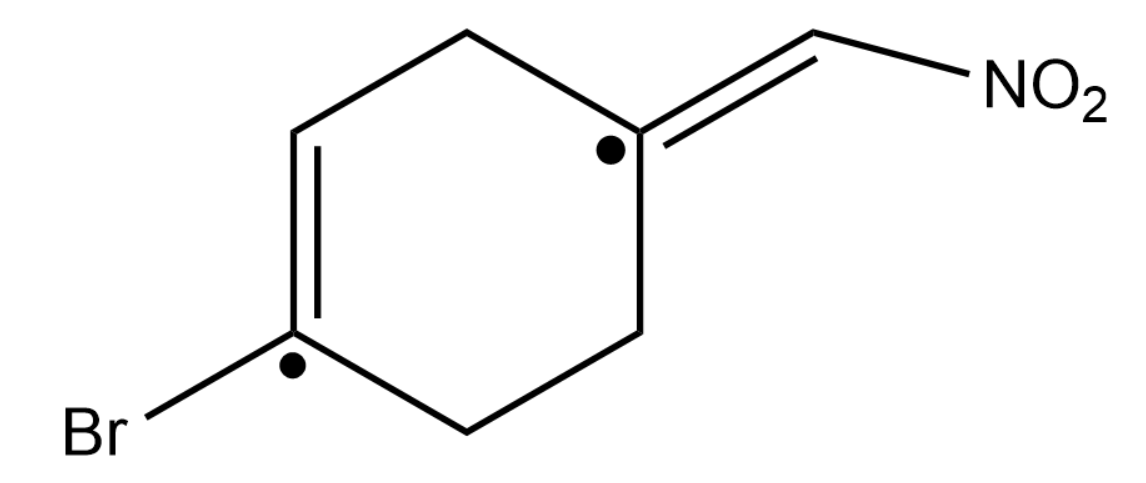
Thus our answer will become
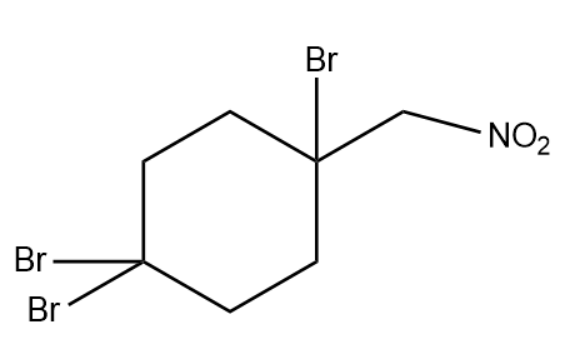
Thus the correct option is (1).
Additional Information:
The main reason for the reaction to proceed this way is due to the formation of stable carbocation.
The most stable carbocation ( 3o ) is formed when hydrogen is attached to the carbon with the most number of hydrogens.
Note :
If there is presence of a peroxide such as Hydrogen peroxide ( H2O2 ) or benzoyl peroxide ( C14H10O4 ) along with the protic acid ( HBr ), then the halogen will get added to the carbon with more number of hydrogens (less substituted carbon). This is exactly the opposite of Markovnikov's Rule. This rule is known as Anti-Markovnikov's Rule. Mechanism for anti-Markovnikov reactions is that of free radical addition.
