Question
Question: Find the product of: \( C{H_2}C{H_2} - O - C{H_2} - C{H_2} - O - C{H_2} - {C_6}{H_5} + H{I_{(Exce...
Find the product of:
CH2CH2−O−CH2−CH2−O−CH2−C6H5+HI(Excess)→?
\left( A \right)OH - C{H_2}C{H_2}OH,{C_6}{H_5}C{H_2} - I,C{H_3} - C{H_2} - I \\\
\left( B \right){C_6}{H_5}C{H_2} - OH,C{H_3}C{H_2} - I,C{H_3}C{H_2} - OH \\\
\left( C \right)I - C{H_2}C{H_2} - I,{C_6}{H_5}C{H_2} - I,C{H_3}C{H_2} - OH \\\
\left( D \right)HO - C{H_2}C{H_2} - OH,{C_6}{H_5}C{H_2} - I,C{H_3}C{H_2} - OH \\\
Solution
In order to solve this question, we are going to first see what mechanism does this reaction favors and which factors are causing the stability of the carbocation that will be formed in this case. Then after writing the reaction equation, for this reaction, we get the products that are obtained from this reaction.
Example of SN1 type nucleophilic reaction.
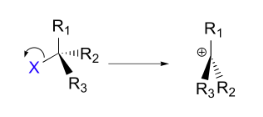
Here, in this example, the C−X bond breaks first, before the nucleophile approaches.
Complete step by step answer:
Presence of excess of HI favors the SN1 mechanism.
Here, SN1 follows a step by step process wherein first, the carbocation is formed from the removal of the leaving group. Then the carbocation is attacked by the nucleophile. Finally the deprotonation of the protonated nucleophile takes place to give the required product. The rate determining step of this reaction depends purely on the electrophilicity of the leaving group and is not impacted at all by the nucleophile.
So, formation of products is controlled by the stability of the carbocation resulting in the cleavage of C−O bond in protonated ether. Thus the product for given reaction are
C6H5CH2I,CH3CH2I,HOCH2−CH2OH
Hence, option (A)OH−CH2CH2OH,C6H5CH2−I,CH3−CH2−I is the correct answer.
Note:
It is important to know that in order to know the products of this reaction, you must know how to differentiate between the SN1 and the SN2 mechanism. SN1 Reactions are unimolecular proceeding through an intermediate carbocation and these reactions give racemization of stereochemistry at the reaction centre. The first step is slower in this case and therefore, determines the rate.
