Question
Question: Find the product A from the given chemical reaction; \( Toulene \xrightarrow{KMn{{O}_{4}}/KOH/{{H...
Find the product A from the given chemical reaction;
TouleneKMnO4/KOH/H2O⊕A
(A) Acetic Acid
(B) Benzene
(C) Benzoic Acid
(D) Benzaldehyde
Solution
Toluene is an organic aromatic compound having a methyl group present on the carbon atom on the benzene ring. First, oxidize the toluene with hot potassium manganate solution and then react it with sodium hydroxide or calcium oxide.
Complete step by step solution:
Toluene is an organic aromatic compound in which the methyl group is present on one of the carbon atoms of the benzene ring. The formula of toluene is C6H5CH3 , Benzene is an aromatic ring of six carbon atoms having three double bonds. The formula of benzene is C6H6
For converting the toluene to benzene in two steps, convert toluene to benzoic acid and then convert this benzoic acid to benzene. When toluene is oxidized with hot potassium manganate solution and potassium hydroxide at 373−383K there is the formation of benzoic acid. Benzoic acid is an organic aromatic compound in which the carbon atom of the benzene ring is attached with the −COOH group. The formula of benzoic acid is C6H5COOH , The reaction is given below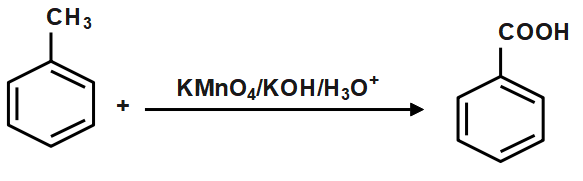
Therefore, correct answer is option C, i.e. Benzoic Acid.
Note:
When the aromatic compounds having methyl groups on the carbon atoms of the benzene ring are oxidized with hot potassium manganate solution, all the methyl groups convert into the acid group, even if more than one methyl is present on the benzene ring.
