Question
Question: Find the Product A and B in the below reaction ...
Find the Product A and B in the below reaction
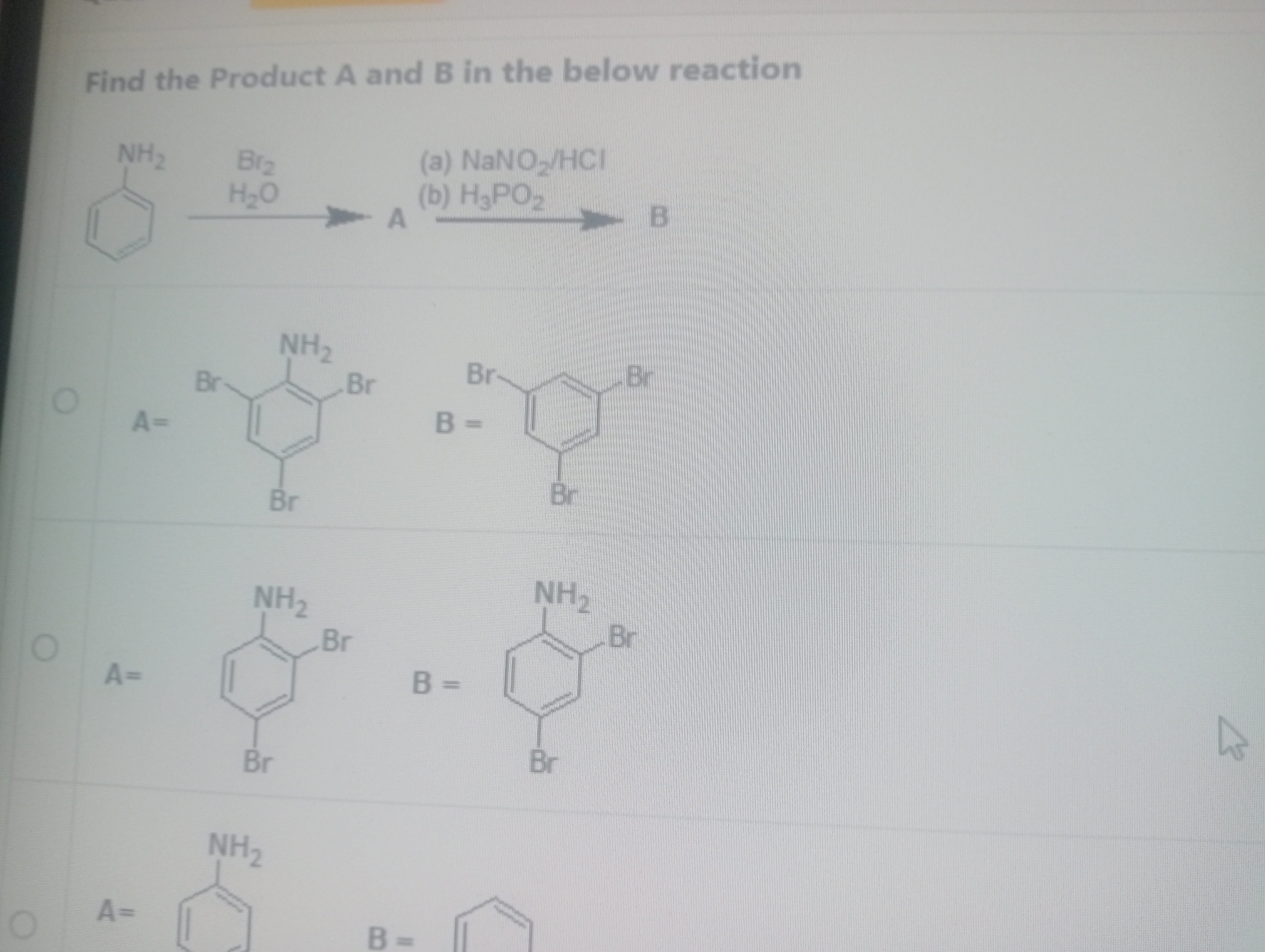
A = 2,4,6-tribromoaniline, B = 1,3,5-tribromobenzene
A = 2,4,6-tribromobenzene, B = 1,3,5-tribromoaniline
A = 2-bromoaniline, B = 3-bromobenzene
A = 4-bromoaniline, B = 1-bromobenzene
A = 2,4,6-tribromoaniline, B = 1,3,5-tribromobenzene
Solution
The reaction sequence involves two main steps: bromination of aniline and then the removal of the amino group.
Step 1: Formation of Product A
- Reactant: Aniline (aminobenzene)
- Reagent: Br2/H2O (Bromine water)
- Mechanism: Electrophilic Aromatic Substitution (Bromination)
The amino group (-NH2) is a powerful activating group and an ortho-para director towards electrophilic aromatic substitution. In the presence of aqueous bromine, which is a highly reactive brominating agent, all available ortho (positions 2 and 6) and para (position 4) positions relative to the amino group are simultaneously brominated. This results in the formation of 2,4,6-tribromoaniline as a white precipitate.
Aniline (C1=CC=C(C=C1)N) reacts with Br2/H2O to give 2,4,6-tribromoaniline.
Product A (2,4,6-tribromoaniline): N(C1=CC(Br)=CC(Br)=C1Br)
Step 2: Formation of Product B
-
Reactant: Product A (2,4,6-tribromoaniline)
-
Reagents: (a) NaNO2/HCl (0-5 °C), (b) H3PO2
-
Step 2(a): Diazotization
The primary aromatic amine (2,4,6-tribromoaniline) reacts with nitrous acid (generated in situ from NaNO2 and HCl) at low temperatures (0-5 °C). This reaction, known as diazotization, converts the amino group into a diazonium group (-N2+). The intermediate formed is 2,4,6-tribromobenzenediazonium chloride.
2,4,6-tribromoaniline (N(C1=CC(Br)=CC(Br)=C1Br)) reacts with NaNO2/HCl to form 2,4,6-tribromobenzenediazonium chloride.
- Step 2(b): Reduction of Diazonium Salt
The arenediazonium salt (2,4,6-tribromobenzenediazonium chloride) is then treated with hypophosphorous acid (H3PO2). Hypophosphorous acid is a reducing agent that replaces the diazonium group (-N2+) with a hydrogen atom. This is a useful method to remove an amino group from an aromatic ring after it has been used to direct other substitutions.
The diazonium group (-N2+) is replaced by a hydrogen atom. The bromines remain on the ring. The numbering of the product is adjusted to give the lowest possible locants to the substituents. Therefore, 2,4,6-tribromobenzene becomes 1,3,5-tribromobenzene.
2,4,6-tribromobenzenediazonium chloride reacts with H3PO2 to give 1,3,5-tribromobenzene.
Product B (1,3,5-tribromobenzene): C1=CC(Br)=CC(Br)=C1Br
Summary of Products:
- Product A: 2,4,6-tribromoaniline
Smiles:
N(C1=CC(Br)=CC(Br)=C1Br) - Product B: 1,3,5-tribromobenzene
Smiles:
C1=CC(Br)=CC(Br)=C1Br
