Question
Question: Find the oxidation number of S in \(HS{ O }_{ 3 }^{ - }\) ion....
Find the oxidation number of S in HSO3− ion.
Solution
Hint: The oxidation number of an atom in a species is the total number of electrons it gains or loses while forming a bond with another atom present in the species. We can find out the oxidation number of an atom in a species using two methods.
Complete step by step solution:
There are two ways of solving this question since they have not mentioned what type of oxidation number. There are two types of oxidation numbers. (a) Calculated or average oxidation number and (b) observed oxidation number. Sometimes the values for both the calculated oxidation number and the observed oxidation number are the same. But they are different for some species. For such species we have to find the actual oxidation number by using the chemical structure of the species.
Calculated oxidation number:
For finding the calculated oxidation number of a particular atom in a species, we have to assume the oxidation number of all other atoms present in the species. The valence number of the atoms with their sign is taken as their assumed oxidation number. For example, if we have to find the oxidation number of hydrogen in H2O, we will assume the oxidation number of oxygen as -2 where 2 is the valency of O atom. Therefore the oxidation number of H will be:
Sumofalltheatoms presentinthespecies=Overallcharge presentonthespecies
Let the oxidation number of H be x,
2x−2=0 (since there are two atoms of hydrogen present in the molecule, therefore x is multiplied by 2. Also the overall charge on the molecule is zero)
⟹x=+1
Now for the species HSO3−, let us assume the oxidation number of S be x. The oxidation number of O and H will be -2 and +1 respectively.
+1+x+[3×(−2)]=−1
⇒x=+4
Therefore the oxidation number of S in HSO3− is +4.
Observed oxidation number:
For calculating observed oxidation numbers we first need to draw the chemical structure of the species.
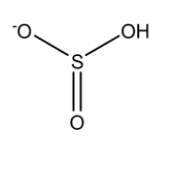
This is the structure of HSO3−
Now, a single bond comprises two electrons, a double bond comprises 4 electrons and a triple bond comprises 6 electrons. So if there is a single bond between the same atoms then the electrons are equally shared between them such that the oxidation number on each atom will be 0. But if is between two different types of atom then the more electronegative atom will attract both of the electrons present in the single bond towards itself such that the oxidation number of the more electronegative atom will be -1 while that of the less electronegative atom will be +1. Since O is more electronegative than S, therefore it will attract all the bonded electrons towards itself.
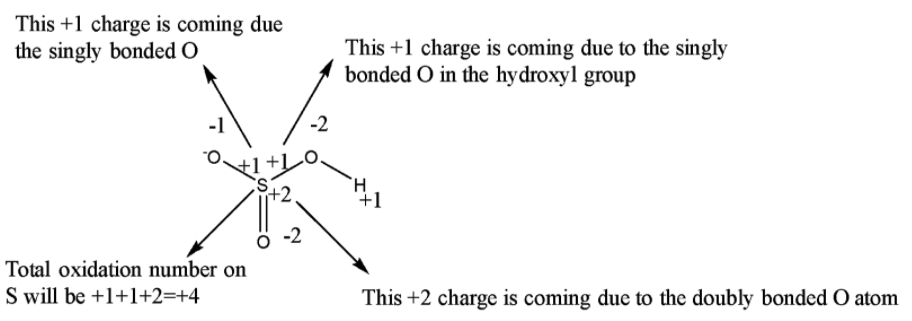
From the above picture, it is clear that the observed oxidation number of S in HSO3− is +4.
Therefore the oxidation number of S in HSO3− is +4.
Note: In the above question, the oxidation of S from both the methods is coming out to be +4. But this is always not the case. Many times the oxidation number of an atom present in a species differs when calculated using both the methods. In such a case, the oxidation number found out by using the second method will correct.
