Question
Question: Find the number of \(S - S\) linkage in \({H_2}{S_2}{O_3}\)....
Find the number of S−S linkage in H2S2O3.
Solution
S−Slinkage is also known as S−S bond. In some cases, it is also called as Disulfide Bridge and is usually derived by the coupling of two thiol groups.
Complete step by step answer:
In biology, disulfide bridges are formed between thiol groups in two cysteine residues. These are a very important component of the secondary and tertiary structure of proteins. In thiosulphuric acid H2S2O3, there are two OH groups which are directly attached to the central atom. It is a basic acid and its dibasic nature supports the S−S bond. The structure of thiosulphuric acid is:
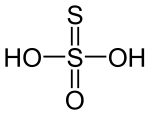
It contains one S−S linkage. Hence, the number of S−S linkage is 1 (one).
Additional information:
The molecular weight of thiosulfuric acid is 114.15g/mol. Thiosulfuric acid is commonly known as sodium thiosulfate. It has the chemical name disodium salt, pentahydrate. IUPAC's name of thiosulfuric acid is sulfurothioic O-acid and its systematic name is dihydroxidooxidosulfidosulfur. It decomposes below 0∘C and it decomposes in water. Its conjugate base is Thiosulfate. Sodium thiosulfate injection is a cyanide antidote and it is used as an adjunct agent for patients taking cisplatin chemotherapy (treatment for various cancers). It is also used in agriculture as an ingredient in chemicals like non- pesticide, fillers, and laboratory chemicals. It can be used as reducing or oxidizing agents.
Note: Sulfurothioic S−acid is a thiosulfuric acid that plays a role as a mouse metabolite. Some students get confused in the number of S−Slinkage i.e. 1 or 2. Remember, it has one linkage with a double bond between two sulfur atoms.
