Question
Question: Find the number of molecules with non-zero dipole moments. 
Solution
Hint : A molecule is said to be having non-zero dipole moment when the distribution of its substituent atoms is such that there is a net resultant force that is not equal to zero and thereby is pointing into certain direction, i.e. either toward or away from the atom. This uneven distribution of polar power/ polarity gives rise to the dipole moment.
Complete Step By Step Answer:
1. In order to find out whether a molecule has a net dipole moment, we need to understand the molecule’s structure and the electronegativity difference between the atoms/groups.
Starting from molecule (a)
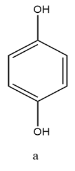
This molecule has a benzene ring and 2 hydroxyl groups attached at the position para to each other.
Now, after considering the structure, let’s see the electronegativity difference. Out of carbon(C) and oxygen(O) , we know that oxygen(O) is more electronegative and thus will pull the shared pair of electrons towards itself and thereby creating a dipole moment whose direction is originating from the carbon atom and towards the hydroxyl group.
After seeing the electronegativity difference, let’s see the distribution of atoms/groups to see the overall distribution of the dipole moment. We can see that the two hydroxyl groups have the same electronegativity, but are in opposite directions to each other.
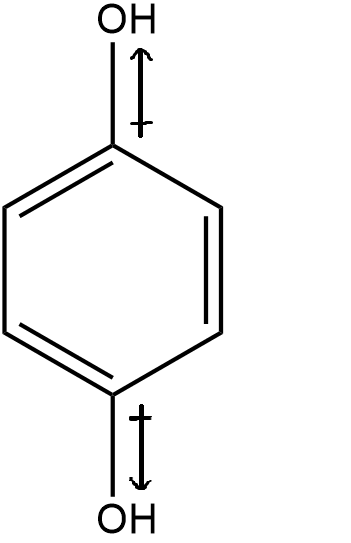
Thereby, it can be deduced that the dipole moment created by both the hydroxyl group will be equal but in opposite direction.
Hence, the net dipole is zero.
Similarly, for the structure (b), we can see that the chloride(Cl) group is present in opposite directions,para to each other.
Similar to hydroxyl, they will have their own individual dipole generation but the overall result will be zero. Hence the molecule has a non zero dipole moment.
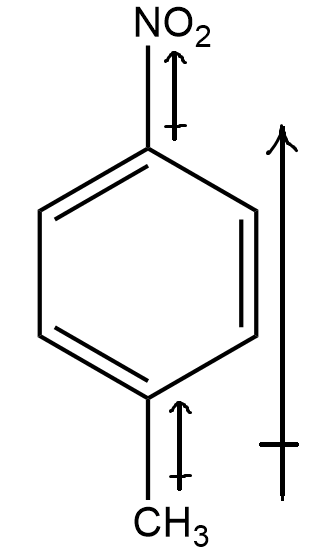
Coming to structure (c)
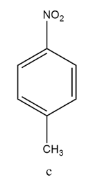
Here, there are 2 different groups, i.e. nitro(NO2) and methyl(CH3) . Seeing the electronegativity of the two group with respect to benzene ring’s carbon (sp2 hybridised) , we know that first, nitro(NO2) group is highly electronegative and also shows –m effect. Hence, the net dipole moment will be towards the nitro group, emerging from the benzene’s carbon.
Secondly, the methyl group is less electronegative than benzene ring’s carbon (sp2 hybridised) , and thus, the resultant dipole moment of this group will be in direction from methyl group to the benzene ring.
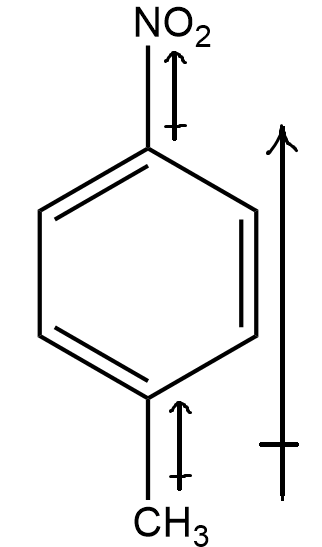
Thereby, combining both, it can be inferred that there will be net dipole moment in the direction from benzene ring to the nitro group. Hence the molecule has non-zero dipole moment.
Structure (d), i.e. pyridine has a sp2 hybridized nitrogen present. Seeing the electronegativity difference, we know that sp2 carbon is less electronegative than the sp2 nitrogen atom.
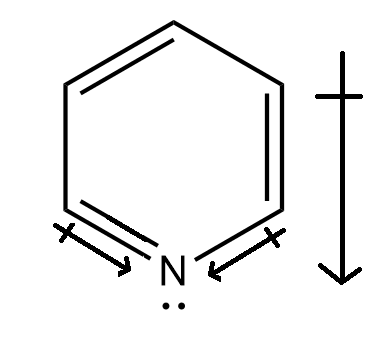
Hence there will be a net electronegative difference and that would result in a net dipole moment. Hence this molecule is having a non-zero dipole moment.
Similarly, just like structure (d), structure (c) also has a net dipole moment.
Structure (f), it has the structure as given below
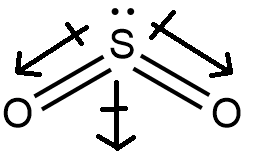
We can see that the molecule is not linear and is bent due to the presence of lone pairs on Sulphur. Considering the electronegativity difference, we know that the oxygen atom is more electronegative than the sulphur atom. This caused a net electronegative difference and thus a dipole moment in the direction from sulphur to oxygen, in downward direction. The vector addition of the two individual dipole moments of oxygen atoms gives the resultant dipole moment which isn’t equal to zero. Hence, the molecule has a non-zero dipole moment.
Structure (g), we can see from its structure
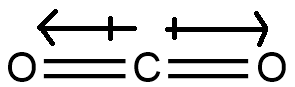
that it is a linear symmetric molecule. Therefore similar to structure (a) and (b), this too will have a net dipole of zero magnitude.
Therefore, the correct options are (c),(d),(e),(f).
Note :
For quick analysis, keep in mind that if a molecule is symmetric and planar, it will not have any dipole moment. Also, keep the relative electronegativity of the elements in mind and then proceed to find the direction of the net resultant dipole moment, if it exists. Also, keep the hybridization of the molecules in mind, because electronegativity varies with hybridization.
