Question
Question: Find the number of lone pairs of electrons on \(Xe\) in \(XeO{F_4}\)....
Find the number of lone pairs of electrons on Xe in XeOF4.
Solution
To find the number of lone pairs of electrons on Xe, we need to draw the structure of XeOF4. We should also be aware of the oxidation states in which the central atom Xe can exist, so as to know the number of electrons involved in bonding and number of lone pairs.
Complete step by step answer:
Xenon is found in multiple oxidation states such as 0, +2, +4, +6 and +8. Due to this it forms a number of oxides, some of these oxides are stable while some are not.
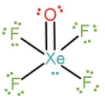
Xenon also forms oxide fluorides which consists of both oxygen and fluorine atoms bonded to Xe. One of such oxide fluorides is xenon oxytetrafluoride, i.e., XeOF4.
The O-atom is in −2 oxidation state and each F-atom is in −1 oxidation state. The overall charge on the molecule is zero, so the oxidation state of Xe is,
x+(−2)+(−1×4)=0
⇒x−2−4=0
⇒x−6=0
⇒x=+6
Xe is a noble gas and it has 8 valence electrons. As it is in +6 oxidation state, it means it has shared 6 electrons with the bonded atoms. Therefore, only 2 more electrons are left in a non-bonded state. That is, one lone pair of electrons is present on Xe.
Structure of XeOF4:
Note:
We can also find the number of lone pairs directly by calculating the number of electrons shared by Xe to the bonded atoms, without the need to calculate the oxidation number. As Xe has 8 valence electrons, it is bonded to O-atom with a double bond, so 2 electrons are shared; 4 F-atoms are singly bonded to Xe, so 4 electrons are shared with fluorine. The rest 2 electrons are present as lone pairs of electrons.
