Question
Question: Find the number of ligand (s) in which the donor atom is \( N \) only. \( \left[ {N{H_2}CO{O^ - }...
Find the number of ligand (s) in which the donor atom is N only.
[NH2COO−, en, dien, py, EDTA]
Solution
Ligand is an ion or molecule that donates electrons and bind to any metal ion present and forms a coordinate complex and generally neutral or anionic molecule has electrons to donate and therefore most of the times ligand is either neutral or anionic. In these ligands given in the question, all of them have Nitrogen atoms but not in all, the donor atom is Nitrogen only and that would be cleared by their structure.
Complete Step By Step Answer:
In the question, a number of ligands are given and from these we have to find out which one has Nitrogen as the main donor atom i.e. in which Nitrogen atom is mainly donating its lone pair and acting as a ligand.
So, a ligand is an ion or a molecule that donates electrons and binds to the central metal ion in a coordination complex. Ligand are generally either neutral molecule or anionic molecule and examples of few ligands are CO, Cl−, H2O, EDTA etc.
So, we will look upon the structure of these ligands in the questions and will see which molecule contains Nitrogen as donating.
In first molecule i.e. NH2COO− we see that oxygen contains negative charge on it and therefore it has excess electrons and will donate lone pairs of electrons from oxygen only. Therefore, in this donor atom is Oxygen.
In second structure i.e. en which is also known as Ethylene diamine and the structure of ethylene diamine is as follows: -
In this molecule we see that it contains Two nitrogen atoms with lone pairs of electrons and when it will get attached to any metal ion, these two nitrogen atoms will donate the electrons.
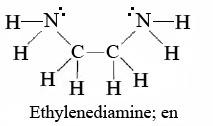
Hence, in this Ethylene diamine, Nitrogen is a donor atom.
In the third structure i.e. dien which means Ethylene diamine and the structure of dien i.e. Ethylene diamine is as follows: -
In this molecule also, there are 3 nitrogen atoms present which will donate its lone pair of electrons when attached to any metal ion.
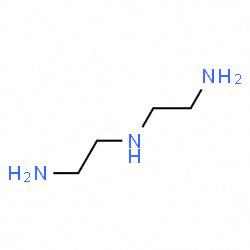
Therefore, in this molecule also Nitrogen will act as a donor atom.
In the next molecule i.e. py which is also known as Pyridine, structure of pyridine molecule is as follows: -
In this molecule also, we see that nitrogen has a lone pair of electrons and will therefore donate when attached to any metal ion. Therefore, Nitrogen will act as a donor atom in this.
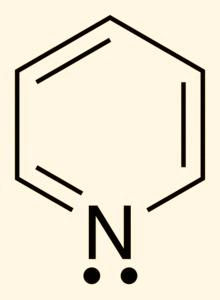
In the next structure i.e. EDTA which is known as Ethylene diamine tetra-acetic acid, the structure is as follows: -
In this molecule, it generally donates and forms coordinate complexes by donating through 4 Oxygen atoms and sometimes from Nitrogen atoms also.
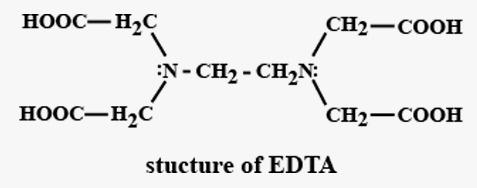
So, it can donate from 4 oxygen and 2 nitrogen atoms.
Therefore, only nitrogen as a donor is not there in this molecule but oxygen is also present.
So, among all the given molecules, Nitrogen is present as the only donor atom in 3 molecules and they are ethylene diamine, ethylene diamine and pyridine.
Note:
Ligands are of different types based on their number of donating atoms present. So, they can be monodentate which has 1 donating atom present or bidentate which has two donating atoms present or tridentate which has three donating atoms or polydentate which has more than 3 donating atoms. In these molecules in question, EDTA is the one which donates either from 4 or 6 donating atoms and forms the complex.
