Question
Question: Find the number of functional groups in the given compound. 
Solution
In organic chemistry, a functional group is a specific group of atoms or molecules which are bonded to compounds at a particular position. These groups account for different characteristic physical and chemical properties of compounds and are responsible to undergo distinctive chemical reactions.
Complete answer:
In the structure of the given compound, the functional groups are represented within the red circle as follows:
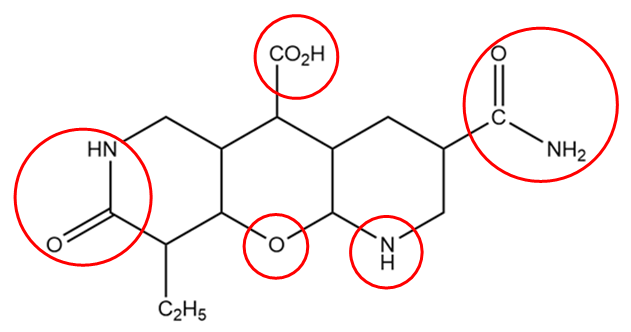
The general representation and name of the functional groups in the given compound are as follows:
1. Primary amide: The general representation of this group is as follows:
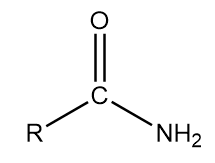
Where, R is an alkyl group and CONH2 part of the structure contributes to the functional group of the compound.
2. Secondary amine: The general representation of this group is R1−NH−R2 , where R1 and R2 are the alkyl groups which can either be same or different and −NH− part of the structure contributes to the functional group of the compound.
3. Ether: The general representation of this group is R1−O−R2 , where R1 and R2 are the alkyl groups which can either be same or different and −O− part of the structure contributes to the functional group of the compound.
4. Secondary amide: The general representation of this group is as follows:
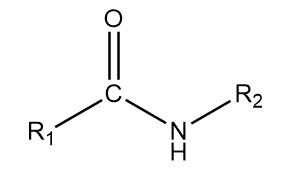
where R1 and R2 are the alkyl groups which can either be same or different and the −CONH− part of the structure contributes to the functional group of the compound.
5. Carboxylic acid: The general representation of this group is as follows:
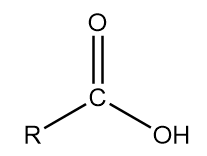
Where, R is an alkyl group and COOH part of the structure contributes to the functional group of the compound.
Hence, the total number of functional groups or substituent groups present in the given compound is =5 .
Note:
Always remember that the alkanes are not considered as a part of functional groups in an organic compound because alkanes consist of carbon-carbon single bonds which have only one sigma bond and are much stronger and stable than alkene and alkyne molecules. Therefore, in the given compound ethyl group i.e., C2H5 group is not considered as a functional group.
