Question
Question: Find the number of bonds between two carbon atoms in \[{\text{Ca}}{{\text{C}}_{\text{2}}}\]....
Find the number of bonds between two carbon atoms in CaC2.
Solution
Draw the structure of CaC2 the molecule. Using the type of bond determines the number of sigma and pi bonds between two carbon atoms in CaC2.
Complete answer:
The formula of a molecule given to us is CaC2. It is calcium carbide.
Carbon can form three types of bonds:
1. Carbon-carbon single bond: A carbon-carbon single bond corresponds to one sigma bond.
2. Carbon-carbon double bond: A carbon-carbon double bond corresponds to one sigma bond and one pi bond.
3. Carbon-carbon triple bond: A carbon-carbon double bond corresponds to one sigma bond and two pi bonds.
To determine the number of bonds between two carbon atoms in CaC2 first we will draw its structure.
The structure of calcium carbide CaC2 is as follows:
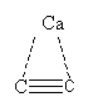
Here, we can see that there is a triple bond between two carbon atoms. As triple bond corresponds to one sigma bond and two pi bonds so we can say that there are 3 bonds between two carbon atoms in CaC2. Out of these 3 bonds, there are one sigma bond and two pi bonds.
Thus, there are one sigma bond and two pi bonds between two carbon atoms in CaC2.
Note:
Calcium carbide is also known as calcium acetylide. In calcium carbide, the cation is calcium and carbide is an anion. To determine the number of bonds it is very important to draw the structure of the molecule correctly. As incorrect structure will give us incorrect numbers of bonds.
