Question
Question: Find the number of acids which are having peroxy linkage from the following: \({{\text{H}}_{\text...
Find the number of acids which are having peroxy linkage from the following:
H3PO5,H2SO5,H2S2O7,H2S2O8,HClO4
Solution
The (−O−O−) is known as peroxy linkage. If direct attachment of oxygen with the central atom, causes the oxidation state of the central atom more than its maximum oxidation state then peroxy linkage forms.
Complete step by step answer:
H3PO5 is known as peroxy phosphoric acid. The oxidation state of the phosphorus in peroxy phosphoric acid is +5. Phosphorus has one double-bonded oxygen atom, two hydroxyl groups and one peroxy linkage.
H2SO5 is known as peroxomonosulphuric acid. It is also known as Caro’s acid. The oxidation state of the sulphur in peroxomonosulphuric acid is +6. Sulphur has two double-bonded oxygen atoms, one hydroxyl group and one peroxy linkage.
H2S2O7 is known as pyrosulphuric acid. It is also known as oleum acid. The oxidation state of both of the sulphur in pyrosulphuric acid is +6. Each sulphur has two double-bonded oxygen atoms, one hydroxyl group and one sulphur-oxygen-sulphur linkage.
H2S2O8 is known as peroxodisulphuric acid. It is also known as Marshall’s acid. The oxidation state of both of the sulphur in peroxodisulphuric acid is +6. Each sulphur has two double-bonded oxygen atoms, one hydroxyl group and one peroxy linkage.
HClO4 is known as perchloric acid. The oxidation state of chlorine in perchloric acid is +7. Chlorine has three double-bonded oxygen atoms and one hydroxyl group.
The structures of all acids are as follows:
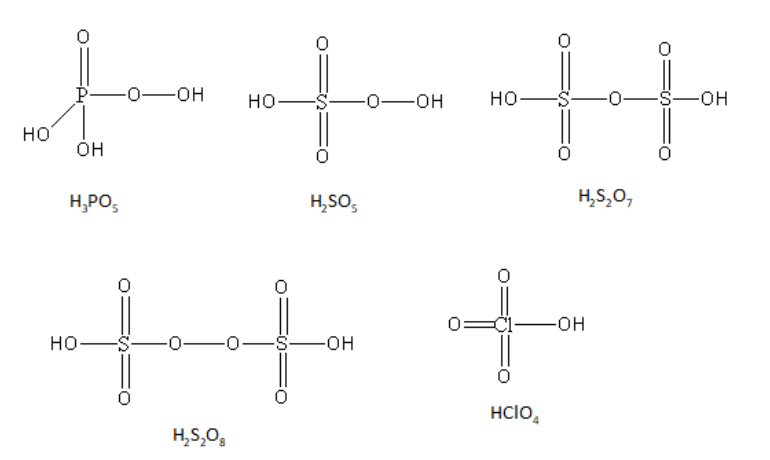
So, the peroxy linkage is present in H3PO5,H2SO5 and H2S2O8.
**Therefore, the acids H3PO5,H2SO5 and H2S2O8 all three have a peroxy linkage.
Note:**
The acids that have peroxy linkage can be identified by the names. The name of acid will start from ‘peroxy’ if the acid has peroxy linkage. O22− is known as peroxide, so this linkage is known as peroxy. The acid which forms by heating of the acid that has a central atom in its maximum oxidation state is known as pyro acid. The pyrosulphuric acid H2S2O7is forms by heating two molecules of sulphuric acid.
