Question
Question: Find the number of acid(s) which are having pyro prefix in its name from the following: \( {H_4}{P...
Find the number of acid(s) which are having pyro prefix in its name from the following:
H4P2O7,H4P2O5,H2S2O7,H2SO5
Solution
There are different prefixes used for the nomenclature of acids. Some commonly used prefixes are hypo-, ortho-, meta-, pyro-, peroxo-, etc.
The prefix pyro- is used when the acid is formed by the combination of two ortho acids involving the removal of one water molecule.
Complete step by step answer:
The prefixes we are discussing here are used for the nomenclature of oxyacids of the same element so as to distinguish between them.
The prefix pyro is used when the acid is formed by the combination of two ortho acids involving the removal of one water molecule. The word pyro is generally used for acids that are formed by the treatment of heat.
The prefix peroxo is used when −O−O− linkage is present in the acid.
Structure of H4P2O7:
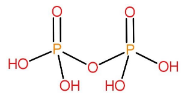
It is formed by the condensation of two H3PO4 molecules.
2H3PO4→H4P2O7+H2O
Name of this acid is pyrophosphoric acid.
Structure of H4P2O5:
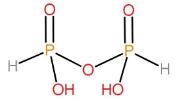
This acid is also formed by the condensation of two ortho acids. Since, it has fewer oxygen atoms than the pyrophosphoric acid, therefore, its name is pyrophosphorous acid.
Structure of H2S2O7:
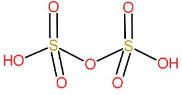
It is formed by the condensation of two molecules of sulphuric acid with removal of one water molecule.
H2SO4+H2SO4→H2S2O7+H2O
Therefore, its name is pyrosulphuric acid.
Structure of H2SO5:
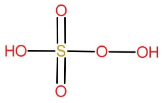
As we can see −O−O− linkage is present, so the name of the acid will have the prefix peroxo. Due to one Sulphur atom it will have the term mono sulphuric in it. So the name of the compound is, peroxymonosulfuric acid.
Therefore, out of the given acids, three acids have the prefix pyro in its name.
H4P2O7,H4P2O5 and H2S2O7.
Note:
The prefix hypo is used for acids which are present in low oxidation states as compared to their corresponding oxyacid.
The prefix meta is used for acids present in their dehydrated forms, while prefix ortho is used for the pure form of the elementos acid concerned, that is, the hydrated form of acid. Ortho acids are the most highly oxidized form of oxyacids.
