Question
Question: Find the major product in the following reaction: 
A) 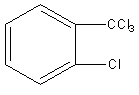
B) 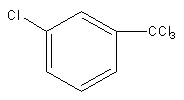
C) 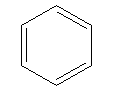
D) 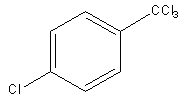
Solution
The reagent Cl2/FeCl3 is used for the chlorination. We can decide the site of chlorination by checking the directing effect of CCl3 group. The CCl3 group is a meta-directing group.
Complete answer:
The chlorine in presence of ferric chloride is used for chlorination. The addition of chlorine is known as chlorination. The chlorination is an electrophilic substitution reaction.
The electrophile chlorine attacks on the benzene ring and then hydrogen removes to give a product.
The chlorination can occur at any position in the benzene ring. But if any group is present on the benzene ring the group causes the partial charge separation, so it directs the attacking group according to its nature on the benzene ring.
CCl3 group has −R effect so, it attracts the electrons towards itself thus, increase the electron density on meta position and decrease the electron density on ortho and para position.
So, electrophile chlorine attacks at the meta position, so the product of the reaction will be meta-chlorinated.
So, the product of the reaction is,
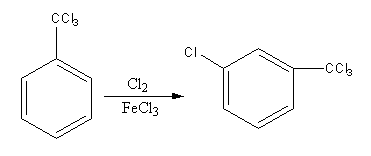
Therefore, option (B) is correct.
Note: The groups that withdraw the electron density form the ring show the −R effect whereas the groups that donate the electron density to the ring show the + R effect. The groups that show−R effect work as meta directing for an electrophile and the groups that show + R effect work as ortho and para directing for an electrophile.
