Question
Question: Find ratio of Speed of sound in $H_e$, Me then, $Co_2$ If ratio of pressure and density are same for...
Find ratio of Speed of sound in He, Me then, Co2 If ratio of pressure and density are same for each gas
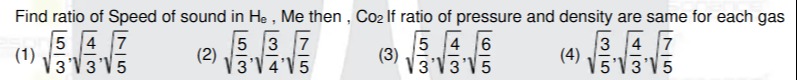
A
35.34.57
B
35.43.57
C
35.34.56
D
53.34.57
Answer
35:34:57
Explanation
Solution
For an ideal gas, the speed of sound is given by
c=ργp,
and since ρp is the same for all gases, the speed depends only on γ.
- Helium (He): γ=35
⇒cHe∝35
- Methane (CH_4) (Me): Being a nonlinear polyatomic molecule, it has 3 translational and 3 rotational degrees of freedom. Thus,
γ=33+2=35 is not correct; the proper calculation is through energy considerations which yield γ=CvCp≈34.
⇒cMe∝34
- Carbon Dioxide (CO_2): It is a linear molecule with 3 translational and 2 rotational degrees of freedom (vibrations not excited at room temperature), so
γ=55+2=57
⇒cCO2∝57
Thus, the ratio of the speeds is
35:34:57.
