Question
Question: Find Product A is: 
a)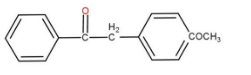
b)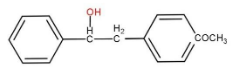
c)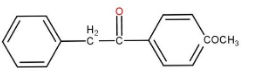
d)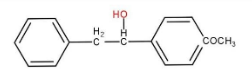
Solution
HgSO4 is a sulphate of mercury having oxidation number of mercury is two. Mercuric sulphate is used as the promoter or enhancer of the electrophilic addition reactions which are having slower rate of electrophilic addition reaction.
Complete step-by-step answer: First of all, we have to know what these reagents do in the reaction.
So, HgSO4 and dilute H2SO4 in combined form, used as in the alkyne hydration and then resulting into the ketones, aldehydes or in the enol forms.
The given reaction is basically used to convert any alkyne into a ketone, alkyne hydration as the intermediate and the formation of the ketone, aldehyde or enol. There is formation of the ketone as the ketone is more stable due to the conjugation of double bond, single bond and double bond.
The reaction as follows:
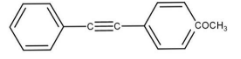 HgSO4dil.H2SO4
HgSO4dil.H2SO4
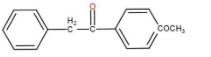
So, the major product is 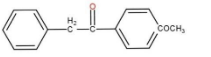
The correct option is (c)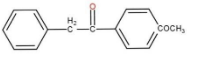
Additional information: Some points about the tautomers:
Enols are the tautomers of the ketones or aldehyde or alcohols. The tautomerism is one of the types of isomerism in which the chemical formula is the same, but the structures are different and they are convertible. So, they are also not considered as true isomers. In tautomerism, there is change in the position of the lone pair and the double bonds to form two different constitutional isomers.
The difference between resonance and the tautomerism:
Resonance is the phenomenon in which displacement of the lone pairs and the double bonds of molecules without affecting the position of the atoms but in the tautomers there is change of the lone pairs and the double bonds to yield the two isomers.
Note: Enols are the intermediate of a chemical reaction having alkene and hydroxyl group at one end of the alkene. The enols are interchanged into ketones and aldehyde to gain the higher stability, although some enols have higher stability than the keto-form, those enols remain in enol form.
