Question
Question: Find out the weight % of carbon in 50 g $CaCO_3$ sample....
Find out the weight % of carbon in 50 g CaCO3 sample.
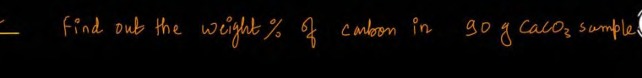
Answer
12.00%
Explanation
Solution
The weight percentage of an element in a compound is calculated by dividing the total mass of that element in one mole of the compound by the molar mass of the compound, and then multiplying by 100. The sample size (50 g) is irrelevant as the percentage composition is an intrinsic property of the compound.
For CaCO3, the molar mass is 40.08(Ca)+12.01(C)+3×16.00(O)=100.09 g/mol. The mass of carbon in one mole is 12.01 g. Thus, the weight percentage of carbon is (12.01/100.09)×100%≈12.00%.
