Question
Question: Find out the number of \[\sigma -\]bonds and \[\pi -\]bonds in benzene....
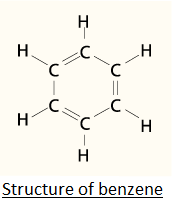
Find out the number of σ−bonds and π−bonds in benzene.
Solution
Hint: To solve these type of questions we need to remember certain points which are as follows:
1. A single bond has 1 σ−bond and 0 π−bonds.
2. A double bond has 1 σ−bond and 1 π−bond.
3. A double bond has 1 σ−bond and 2 π−bonds.
Complete step by step solution:
In case of benzene, Benzene has a molecular formula which means there is presence of a double bond in between all alternate carbon atoms and each carbon is attached to two more carbon atoms by means of a single bond and a double bond and also a hydrogen atom by a single bond. So in total we have 9 single bonds and 3 double bonds. As we know a single bond has 1 bond and 0 π−bonds, a double bond has 1 σ−bond and 1 π−bond a double bond has 1 σ−bond and 2 π−bonds. So there are a total of 12 sigma bonds and 3 pi bonds in benzene.
Note: Sigma and pi bonds are types of covalent bonds which differ in the overlapping of atomic orbitals. Covalent bonds are formed by the overlapping of atomic orbitals between the atoms. Sigma bonds are a result of the head-to-head overlapping of atomic orbitals whereas pi bonds are formed by the lateral or sideways overlap of two atomic orbitals.
